Abstract
This paper reports the first published prevalence of Multiple endocrine neoplasia type 2B (MEN 2B). Estimates of the epidemiology of MEN 2B were based on a single population with one centralized genetic clinic. MEN 2B remains a relatively rare condition and can be easily missed if the diagnosis has not been considered.
Introduction
Multiple endocrine neoplasia type 2 (MEN 2) is a hereditary autosomal dominant endocrine syndrome comprising MEN 2A, MEN 2B, and familial medullary thyroid cancer (MTC) [1, 2]. MEN 2B is characterized by early development of aggressive MTC, and prophylactic total thyroidectomy is advocated by 6 months of age [3–5]. Multiple and bilateral pheochromocytoma occur in 50% of patients, and some with undiagnosed pheochromocytoma may die of cardiovascular crisis perioperatively [4, 6].
MEN 2B may be identified in infancy or early childhood [7] by the presence of mucosal neuromas of the anterior dorsal surface of the tongue, palate, or pharynx. Identification of RET gene mutations in MEN 2B in 1994 allowed the possibility of direct DNA-based mutation testing [3]. Before that, linkage testing was available [8, 9]. RET molecular genetic testing should be performed as soon as possible after birth in all children known to be at risk or, if no family history exists, as soon as the clinical diagnosis is suspected [2]. Most patients have mutations in codon 918 and, rarely, in codons 883 and 922.
The epidemiology of MEN 2B is unknown. The prevalence of all MEN 2 cases is ∼1 in 35,000 [2]. The prevalence of MEN 2B is estimated as between 1 in 600,000 [10] to 1 in 4 million [11], but no figures exist. The annual incidence has been estimated at 4 per 100 million per year [12].
Methods
The Northern Ireland regional genetics service covers a fixed population of ∼1.8 million and has a centralized network of genetic clinics covering the entire region, with a single molecular genetics laboratory. The population is stable, with little immigration and emigration, and is ideal for epidemiological studies. Two-thirds of the region is bound by sea and the remainder by a land border with the Republic of Ireland. Prospective records have been maintained for MEN 2 cases since 1988. We interrogated the register of MEN 2B cases to identify cases. Molecular genetic confirmation of diagnoses was carried out in all cases. We included cases with no definite or substantiated family history, all confirmed as MEN 2B on genetic testing, and referred to the genetic clinic between 1988 and 2012. All patients were clinically examined, and a detailed history of symptoms, investigations, and procedures undertaken as well as family history were obtained and validated by medical records.
Inclusion criteria for testing included MTC at an early age, marfanoid body habitus, or presence of mucosal neuromas or other characteristic features of MEN 2B.
For the prevalence calculation, period prevalence and point prevalence were calculated. Point prevalence was calculated on the prevalence day of April 21, 2012. Period prevalence was calculated from 1988 to 2012 (average population of 1,689,588 and 4 cases). Period prevalence was defined as persons with a given health indicator during a specified time period in a population during the same time period.
The total population studied was recorded at the last census on April 21, 2012 (1.824 million) [13].
Results
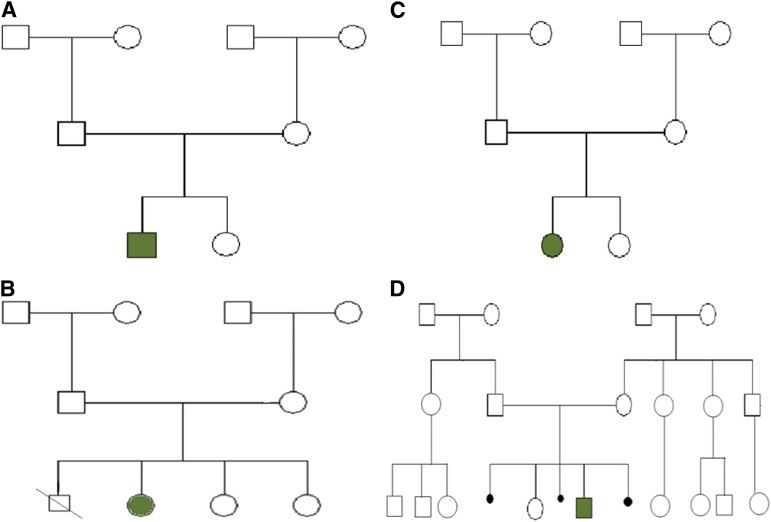
The period prevalence was 0.178 × 10−5 (mean 24 year population: 1,689,588). The point prevalence was 0.219 × 10−5. Four sporadic cases were identified throughout 24 years. Three patients were alive on the prevalence day, April 21, 2012. Clinical features are shown in Table 1, and family trees are shown in Figure 1.
Table 1.
Clinical features of the multiple endocrine neoplasia type 2B cases

Figure 1.
Pedigrees of families with multiple endocrine neoplasia type 2B (A–D). Affected index cases are shown in green shading.
All of the patients had MTC: two in childhood (aged 8 and 10 years) and two in adulthood (aged 24 and 37 years). Three were diagnosed with pheochromocytoma. The youngest patient remains under thorough screening and remains symptom free to date. All had elongated faces with wide-eyed expressions, large eyebrows, and prominent lips and mucosal neuromas (two patients underwent cosmetic surgery to have neuromas removed from their lips). All had a typical marfanoid body habitus. Three had some gastrointestinal problems including constipation.
Discussion
This paper reports the first published prevalence of MEN 2B. Estimates of the epidemiology of MEN 2B were based on a single population with one centralized genetic clinic.
We sampled a stable population in a defined geographical area, and 100% of cases had the common codon 918 mutation. Ascertainment is fairly close to complete with rigorous register information on cases since 1988, giving minimum prevalence because mild cases may still be missed.
We have excellent relationships with the centralized endocrine medical and surgical teams that refer all cases of medullary thyroid cancer for assessment.
Defining the prevalence of MEN 2B is an important step in determining the public health impact of this condition. Accurate figures allow prediction of numbers of expected cases in populations.
The clinical features of our patients are all consistent with MEN 2B. None of the MEN 2A cases examined in this period had evidence of neuromas or early onset of MTC. The codon 918 mutation was present in all of our patients and is the most common mutation. Cascade screening confirmed the mutation to be de novo in all of our cases. No cases due to the rare codon 883 or 922 mutations were identified, and cases with these codon mutations appear to have a similar phenotype to those with the common 918 mutation. In all cases, early prophylactic thyroidectomy is the treatment of choice before 6 months of age; given the aggressive progression in some cases, consideration should be given to thyroid surgery within the first month of life [14]. Planned surgery after testing of cord blood or prenatal testing may be helpful in offspring of those with diagnosed cases; however, recognition of cases in early life may be difficult because all our cases were de novo mutations.
Conclusion
The prevalence of MEN 2B is ∼0.2 × 10−5. All MEN 2B cases were new mutations with no positive family history, and all presented classically with MTC, marfanoid body habitus, and mucosal neuromas. All had the common codon 918 mutation in exon 16 of RET. MEN 2B remains a relatively rare condition and can be easily missed if the diagnosis has not been considered.
Footnotes
For Further Reading:Madhuchhanda Roy, Herbert Chen, Rebecca S. Sippel. Current Understanding and Management of Medullary Thyroid Cancer. The Oncologist 2013;18:1093–1100.
Implications for Practice:Medullary thyroid cancer (MTC) typically accounts for approximately 4% of all thyroid cancers. Of these, 20% of cases are hereditary and can be found in multiple endocrine neoplasia syndrome or as part of familial MTC based on a specific germline mutation in the RET proto-oncogene. This article summarizes the current approaches and guidelines available for the diagnosis, evaluation, and management of patients and their family members with suspected MTC. Surgery is the standard of care, and prophylactic surgeries are performed in genetic carriers. Tyrosine kinase receptor inhibitors vandetanib (ZD6474) and cabozantinib (XL184) were recently approved by the U.S. Food and Drug Administration as promising systemic therapy for advanced MTC.
Disclosures
The authors indicated no financial relationships.
References
- 1.Gertner ME, Kebebew E. Multiple endocrine neoplasia type 2. Curr Treat Options Oncol. 2004;5:315–325. doi: 10.1007/s11864-004-0022-6. [DOI] [PubMed] [Google Scholar]
- 2.Moline J, Eng C. Multiple endocrine neoplasia type 2. Available at http://www.ncbi.nlm.nih.gov/books/NBK1257/. Accessed July 28, 2014.
- 3.Morrison PJ, Nevin NC. Multiple endocrine neoplasia type 2B (mucosal neuroma syndrome, Wagenmann-Froboese syndrome) J Med Genet. 1996;33:779–782. doi: 10.1136/jmg.33.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng C, Smith DP, Mulligan LM, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Morrison PJ, Atkinson AB. Genetic aspects of familial thyroid cancer. The Oncologist. 2009;14:571–577. doi: 10.1634/theoncologist.2009-0046. [DOI] [PubMed] [Google Scholar]
- 6.Morrison PJ, Hadden DR, Russell CFJ, et al. MEN2A: An update on the Northern Ireland pedigree. Henry Ford Hosp Med J. 1989;37:127–128. [PubMed] [Google Scholar]
- 7.Millar S, Bradley L, Donnelly DE, et al. Familial pediatric endocrine tumors. The Oncologist. 2011;16:1388–1396. doi: 10.1634/theoncologist.2011-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison PJ, Hadden DR, Hughes AE, et al. Gene probe analysis in an informative family with multiple endocrine neoplasia syndrome type 2A (MEN 2A). Improvement in carrier risk estimations. Q J Med. 1991;78:597–603. [PubMed] [Google Scholar]
- 9.Morrison PJ, Nevin NC, Hughes AE, et al. Presymptomatic screening for MEN-2A. Lancet. 1991;337:299. doi: 10.1016/0140-6736(91)90908-8. [DOI] [PubMed] [Google Scholar]
- 10.Marx SJ. Multiple endocrine neoplasia. In: Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Elsevier/Saunders; 2011. pp. 1728–1767. [Google Scholar]
- 11.Moline J, Eng C. Multiple endocrine neoplasia type 2: An overview. Genet Med. 2011;13:755–764. doi: 10.1097/GIM.0b013e318216cc6d. [DOI] [PubMed] [Google Scholar]
- 12.Kebebew E, Gosnell JE, Reiff EP. Multiple endocrine neoplasia type 2B. In: Ruggieri M, Pascual-Castroviejo I, Di Rocco C, editors. Neurocutaneous Disorders Phakomatoses and Hamartoneoplastic Syndromes. New York, NY: Springer; 2008. pp. 695–701. [Google Scholar]
- 13. Census 2011, key statistics for Northern Ireland: December 2012. Available at http://www.nisra.gov.uk/Census/key_report_2011.pdf. Accessed July 31, 2014.
- 14.Gómez I Gavara C, Ponce Marco JL, Belda Ibañez T, et al. The optimal age for performing surgery on patients with MEN 2B syndrome. Oncol Lett. 2011;2:929–930. doi: 10.3892/ol.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]