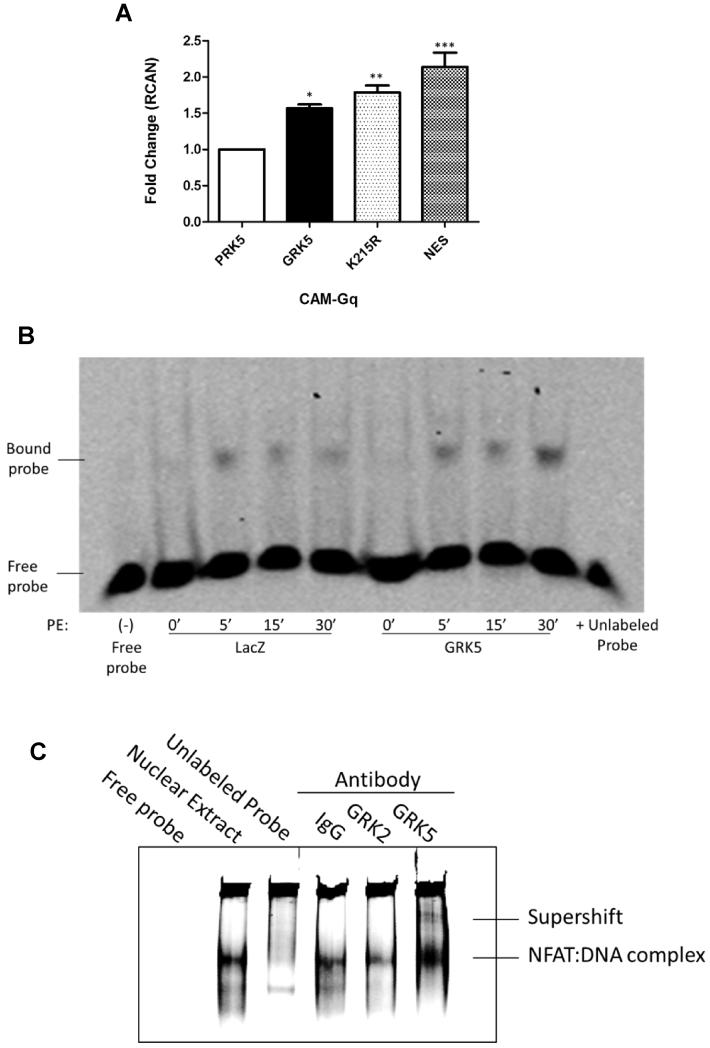
Figure 5. GRK5 Interacts with NFAT in a Kinase-independent Manner Through DNA Binding.
(A) Quantitative RT-PCR for NFAT target gene RCAN in H9c2 cells transfected with wild-type GRK5, kinase dead (K215R) GRK5 mutant, nuclear export sequence (NES) GRK5 mutant or vector (pRK5) control for 72 hours and infected with CAM-Gq virus for 24 hours. (n=3; *, p<0.05; ** p<0.01; ***, p<0.01 by ANOVA). (B) Electrophoretic mobility shift assay was carried out with 2 μg of nuclear extract (NE) from cultured NRVMs infected with GRK5 or LacZ control and stimulated with hypertrophic agonist phenylephrine (PE) for 0, 5, 15 or 30 minutes to analyze DNA binding activity of NFAT using IR dye-labeled oligonucleotides containing the consensus NFAT binding sequence. Lane 1 is free probe no NE; lanes 2 to 9 GRK5 overexpression and 50μmol/L PE treatment as indicated; lane 10 NE from GRK5 overexpressing myocytes stimulated with 50μmol/L PE for 30 minutes and 200-fold excess non-labeled consensus oligonucleotides. Shown is a representative image from 3 independent experiments. (C) Antibody-mediated super-shift EMSA using 5μg NE from AdGRK5 infected (NRVMs) stimulated with PE for 30 minutes. Samples were incubated with 1 μg specific antibodies to GRK2, GRK5 or rabbit IgG. Shown is a representative image from 3 independent experiments.