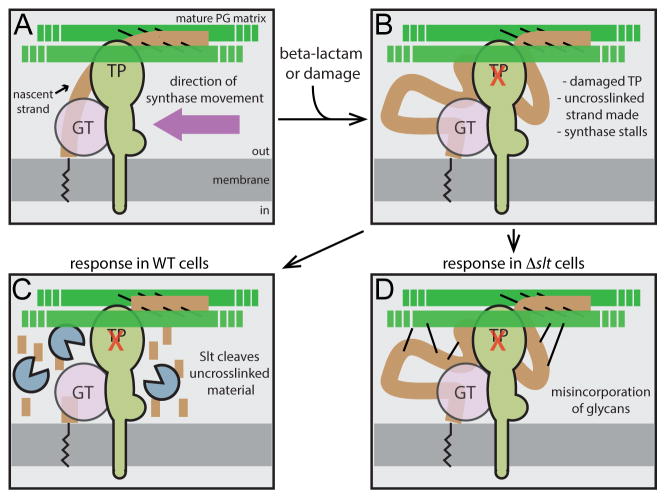
Figure 7. Role of Slt in maintaining coordination between GT and TP activity during PG biogenesis.
Shown is a schematic highlighting the role of Slt in PG biogenesis. Panel (A) shows the normal synthetic process with properly coupled GT and TP activities. The new glycan strands (brown) are polymerized from lipid-linked precursors (black zig-zag line) by an enzyme with GT activity (pink) that may either be the canonical domain of an aPBP or an as yet unidentified GT enzyme. The newly polymerized glycans are rapidly crosslinked into the mature matrix (green) by an associated TP activity shown here as a bPBP, but it could also be the TP domain of an aPBP. Other components of the putative synthetic complex including cytoskeletal elements were omitted for simplicity. When the TP active site is damaged or targeted by a beta-lactam (panel B), GT activity continues to produce glycan chains that are no longer crosslinked into the matrix. In WT cells (panel C), such strands are targeted for destruction by Slt. Conversely, in cells inactivated for Slt (panel D), the uncrosslinked glycans produced by the damaged/targeted machinery are not degraded, but are instead aberrantly incorporated into the matrix by an alternative crosslinking enzyme. In cells with normal FtsZ levels, the resulting morphological changes are lethal, leading to beta-lactam hypersensitivity of Slt-defective cells.