INTRODUCTION
Nature of the Problem
Cerebral palsy (CP) is a motor disorder caused by a nonprogressive injury to the developing brain.1 The injury occurs perinatally and, though causes are rarely known,2,3 CP is common in infants born preterm with small birth weights.4 CP occurs in 2 to 3 of every 1000 live births5 and has heterogeneous symptoms, anatomic involvement, and functional impairment, including lifelong changes in motor function.1,2 These alterations stem from both changes in the neural drive to muscles6 and changes to muscles themselves.
Symptoms
Spastic CP, which involves injury to the pyramidal system, is the most common form of CP, making up nearly 75% of all cases.3 Spasticity has been defined as a “velocity dependent resistance to stretch.”7 Limb involvement varies, with patients showing symptoms in either all 4 limbs (tetraplegia or quadriplegia), primarily on one side of the body including one upper and lower extremity (hemiplegia), or primarily in the lower extremities (diplegia).8 Patients’ functional mobility can be classified using several rating scales, including the Gross Motor Function Classification System (GMFCS), which rates patient mobility on a scale of 1 to 5 from high to low function, respectively.1,3 Although the injury associated with CP initially occurs in the developing brain, symptoms are commonly treated at the muscle level. Because the population affected with CP is large and heterogeneous, a better understanding, especially among clinicians and therapists, of muscular adaptations in CP may lead to improvements in treatment or even development of completely novel therapeutic strategies.
To understand the adaptations that occur in muscle from CP patients, it is important to review the function of typically developing muscle.
HEALTHY SKELETAL MUSCLE STRUCTURE AND FUNCTION
Muscle Structure
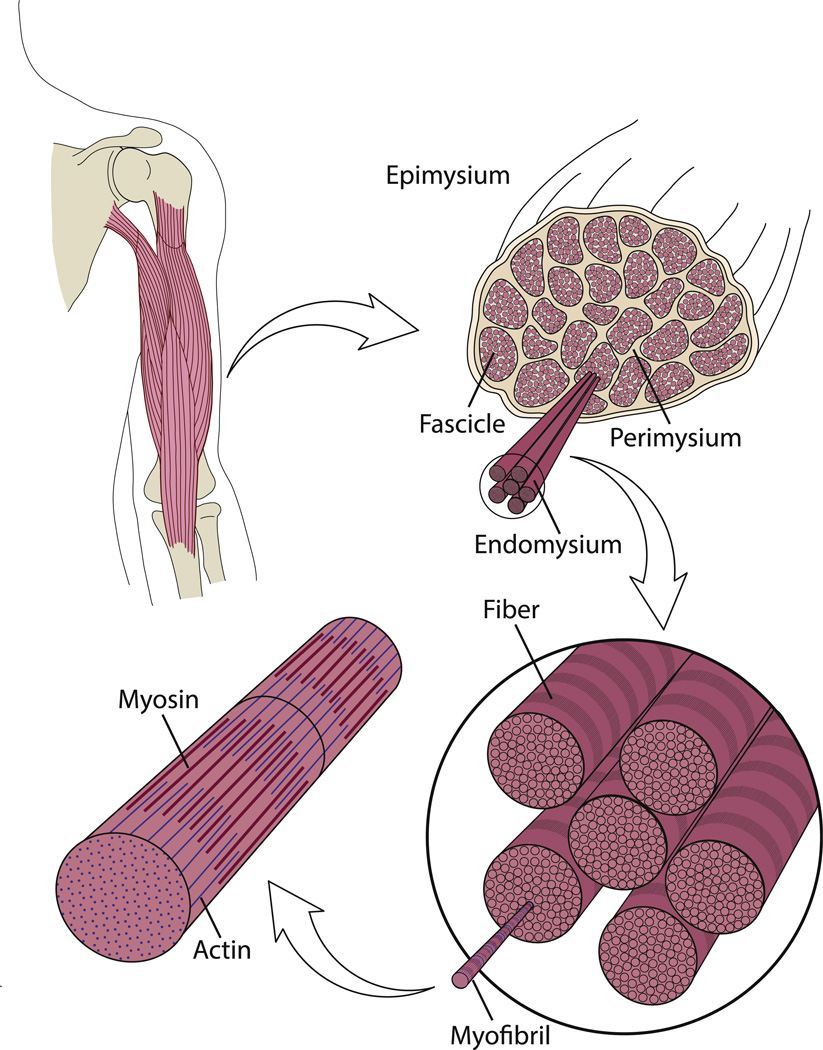
The fundamental unit of muscle force production is the sarcomere. Sarcomeres produce force by the interaction between 2 proteins, actin and myosin. Force production is affected by both muscle velocity and the amount of overlap between these 2 proteins, or sarcomere length. The sarcomere length-tension relationship has been characterized in the length-tension curve.9 Sarcomeres are joined end to end (in series) to form myofibrils. Bundles of myofibrils form myofibers, or multinucleated muscle cells. These muscle fibers are joined into muscle fiber bundles, or fascicles (Fig. 1).
Fig. 1.
Structural hierarchy of skeletal muscle. Skeletal muscle is composed of bundles of muscle fibers called fascicles. Individual fibers in these fascicles consist of myofibrils, which are composed of the contractile proteins actin and myosin. Connective tissue, which surrounds the muscle at many levels, is organized into epimysium, surrounding the whole muscle; perimysium, surrounding fascicles; and endomysium, surrounding muscle fibers.
At each increasing size scale, extracellular matrix (ECM), the surrounding connective tissue, encapsulates muscle structures. Endomysium surrounds individual myofibers,10 perimysium surrounds muscle fascicles,11 and epimysium surrounds the whole muscle (see Fig. 1).12,13 The composition and arrangement of these structures is important to muscle function, and can vary in muscle disorders.
The extensive growth and regeneration capacity seen in muscle is due to its intrinsic stem cell population. Most of these stem cells are called satellite cells14 and are found below the basal lamina of myofibers; they are normally quiescent except when activated during times of muscle disease or injury.15 Satellite cell number and viability, rather than being constant throughout life, decreases with age or diseases that are characterized by extensive regeneration.16 Conditions such as muscular dystrophy, which require constant regeneration of muscle fibers, are believed to eventually lead to exhaustion of the satellite cell population17 and the concomitant loss in a muscle’s ability to adapt to the new functional demand.
Plasticity
Muscle has strong regenerative capacity, and can respond and change based on functional demands; for example, muscle fiber atrophy (leading to a decrease in muscle fiber size) when subject to decreased use, aging, and some diseases. Serial sarcomere number can also change in response to growth18 as well as limb immobilization with the muscle in a shortened or lengthened position. This serial change in sarcomere number resulting from chronic change in muscle length was shown in several classic studies in both mouse19 and cat.20 These muscles, immobilized in a shortened position, rapidly adjust their sarcomere number to restore sarcomere length to previous values. A similar response was reported in a human case study of distraction osteogenesis in which a leg-length discrepancy was corrected as a patient’s bone was web 4C=FPO gradually lengthened over time.21 Sarcomere lengths were measured and sarcomere number calculated over the course of the treatment. As stretching of the bone and muscle occurred, sarcomere number rapidly increased and sarcomere length nearly returned to the pretreatment value.21 Serial sarcomere number from patients with CP appears to be altered in comparison with typically developing muscles, however, suggesting that the plasticity seen in typically developing muscles may not be present to the same extent in CP.
MUSCLE PATHOLOGY OF CEREBRAL PALSY
Force Production and Muscle Function
Alterations in gait, balance, and force production have been reported for patients with CP.22–26 For example, knee extensor force decreases with CP, which can significantly inhibit mobility.22 Voluntary force production in general is decreased, as shown by many investigators.27–29 There is also evidence that greater cocontraction, or simultaneous activation of a muscle and its antagonist, occurs in CP.30 Compounding the problem of decreased force production, ankle stiffness was also shown to be 51% higher in CP, indicating increased resistance to passive ankle flexion.31
Muscle Architecture
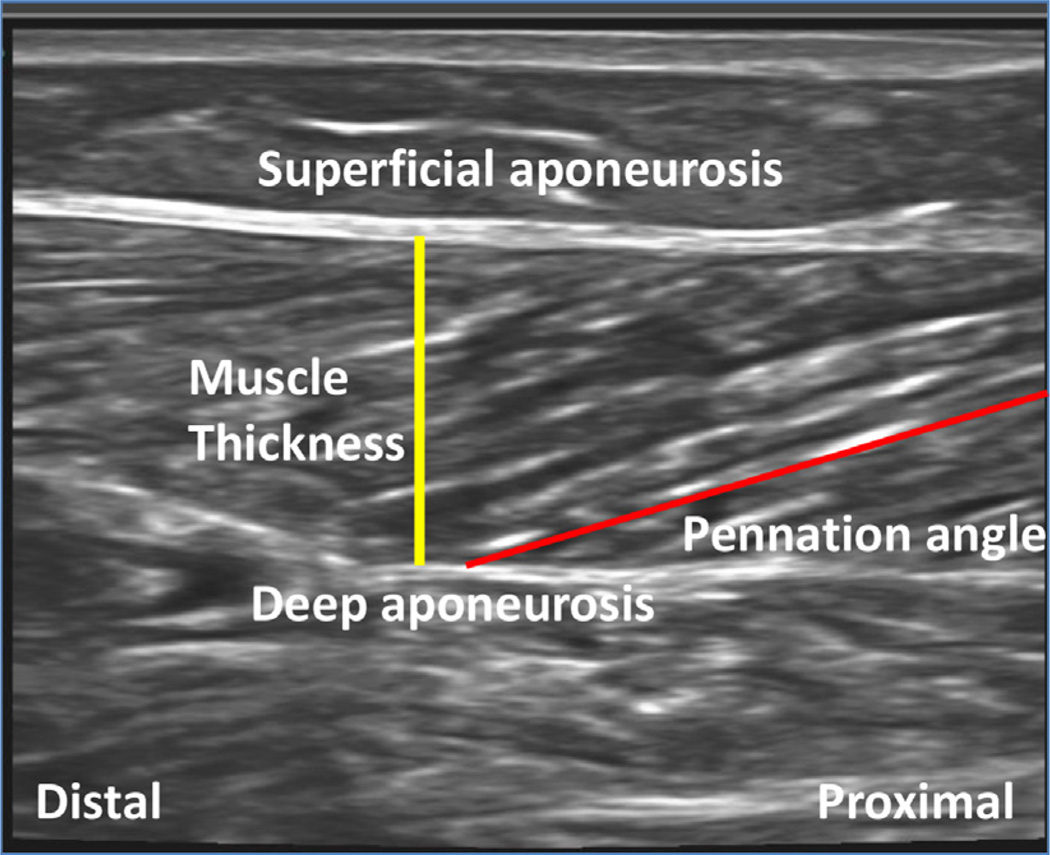
Muscles in spastic CP often develop contractures, whereby joint range of motion is limited and muscles appear functionally “short.”32 Many researchers have measured changes in muscle properties such as muscle belly size, muscle length, fascicle length, and sarcomere length, all of which may help to explain this observation. Ultra-sonography is probably the most common tool used to describe basic muscle structural changes, such as fiber length and tissue thickness (Fig. 2).33 Previous architecture studies focused primarily on the gastrocnemius muscle, an ankle plantar-flexor and knee flexor that is commonly implicated in ankle equinus contractures34 and also plays a role in knee flexion contractures. Ultrasonographic measurements show that gastrocnemius muscle volume is smaller in patients with CP. When affected and unaffected limbs were compared in hemiplegic patients, muscle volume was print & web 4C=FPO decreased by 28% in the affected gastrocnemius and by nearly 50% more than muscles of typically developing children.35 Muscle belly lengths have also been shown to decrease in CP. One 3-dimensional ultrasonography study of the medial gastrocnemius showed decreased muscle belly lengths, but no change in fascicle length when normalized to tibia length.35 Based on the description of muscle structure presented herein, it is clear that ultrasonography alone cannot make functional predictions based on gross tissue dimensions because the composite sarcomeres cannot be detected using this modality.
Fig. 2.
Typical ultrasonography image of a human soleus muscle. Muscle appears dark and the connective tissue light on the sonogram. Superficial and deep aponeuroses surround the muscle on either side. The red line indicates the path of a muscle fascicle and the yellow line represents the thickness of the muscle belly.
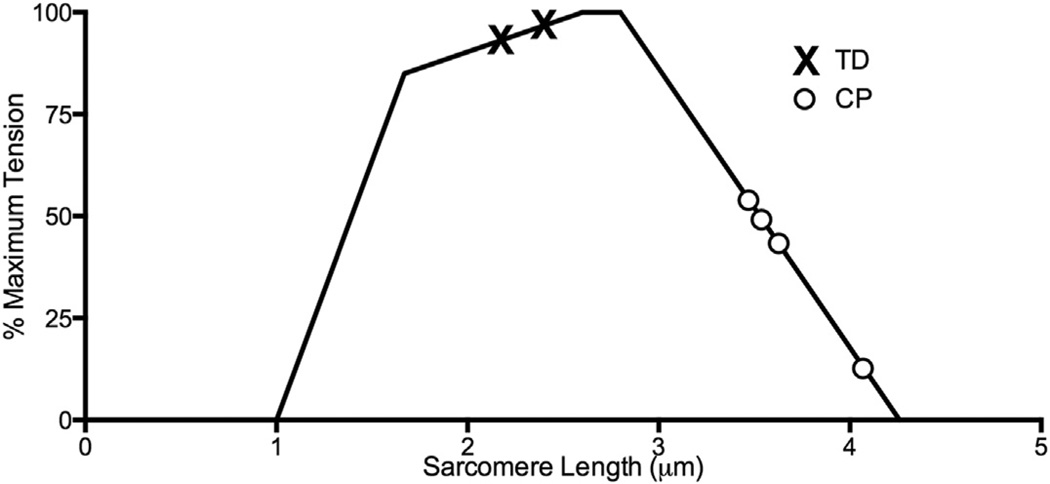
In contrast to what might be expected for contractures, which are often considered permanently contracted or shortened muscles, previous fascicle length measurements in patients with CP have been inconclusive. Whereas some studies report shorter muscle fascicles in CP,34,36 others report no difference between typically developing and CP fascicle lengths.35,37 In contrast to the variety of results reported for fascicle length, sarcomere length, the best predictor of active muscle force, has been consistently shown to be longer in CP patients (Fig. 3). Whereas one study questioned the functional significance of sarcomere lengthening based on force measurements across the joint range of motion,38 previous direct studies of sarcomere length show long sarcomeres in CP in both upper39 and lower extremity flexors.40 It therefore appears that regulation of sarcomere length does not occur similarly in patients with typical development and those with CP, as CP sarcomeres do not maintain a relatively constant length as in normal development.
Fig. 3.
Schematic length-tension curve of skeletal muscle. When sarcomere length data for cerebral palsy (CP; O symbols) and typically developing (TD; X symbols) muscles are plotted on this curve, sarcomeres clearly act in a fundamentally different region of the curve for CP in comparison with TD subjects. For example, if TD sarcomeres are stretched, maximal force production will increase, whereas the opposite will happen for CP sarcomeres. Muscles represented include gracilis, semitendinosus, soleus, and flexor carpi ulnaris. (Data from Mathewson MA, et al. submitted; and Lieber RL, Fridén J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 2002;25(2):265–70; and Smith LR, Lee KS, Ward SR, et al. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 2011;589(10):2625–39.)
TISSUE PROPERTIES
Mechanical Properties
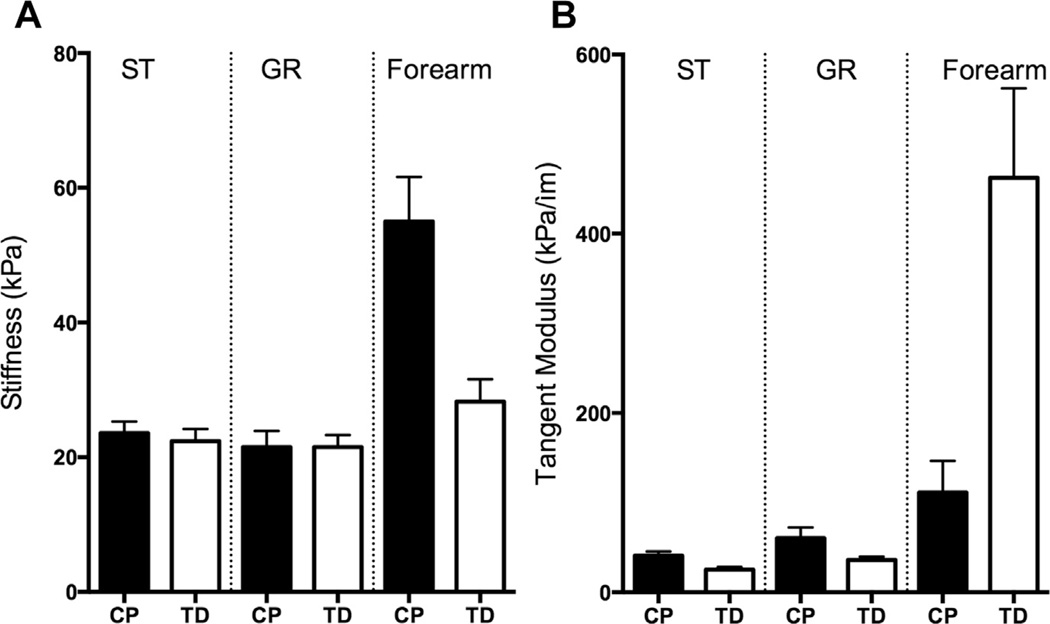
While CP contractures are often thought to be stiff because of muscle overactivation, there are also critical contributions to stiffness that simply result from increased intrinsic passive stiffness of the tissue. A recent study explored the passive mechanical properties of 2 lower extremity muscles, the semitendinosus and gracilis.40 Fibers, whose passive mechanical properties are thought to depend mainly on the giant structural protein titin, showed similar stiffness in typically developing and CP tissue of both muscles. Titin mass was no different between the 2 groups, as would be expected from the similar fiber stiffness. Gracilis bundles were much stiffer than either typically developing bundles or bundles from the CP semitendinosus. CP semitendinosus bundles were also stiffer than their typically developing controls (Fig. 4). This difference in stiffness was likely due to the contributions of the ECM at the bundle level. The change in bundle stiffness between typically developing and CP tissue suggests that collagen content has increased or that there is some type of abnormal organization in the muscle ECM. Although increased collagen was observed in these muscles,40 there are currently no tools available to quantify muscle extracellular structures accurately. Of interest, earlier studies in the upper extremity yielded very different results, reporting stiffer fibers41 but more compliant bundles in patients with CP,42 even though the ECM in CP muscle bundles tested in this study occupied more space than the ECM of typically developing controls.42 The differences in stiffness among muscles, potentially resulting from differences in ECM quality and arrangement, highlight the importance of studying muscles individually rather than making generalizations, especially in the case of highly heterogeneous disorders such as CP. In addition, it is important to develop new tools that can allow accurate measurement of muscle properties that have the greatest clinical relevance.
Fig. 4.
Comparison between fiber and bundle stiffness in children with cerebral palsy (CP) and typically developing (TD) children. Note that axes are not the same for A and B. (A) Although fibers between CP and TD individuals are similar in stiffness in the gracilis (GR) and semitendinosus (ST), they are stiffer in the forearm muscles of individuals with CP. (B) Bundles of muscle fibers and their surrounding extracellular matrix were stiffer in CP in the GR and ST, but less stiff in the forearm. (Data from Refs.40–42)
Histology
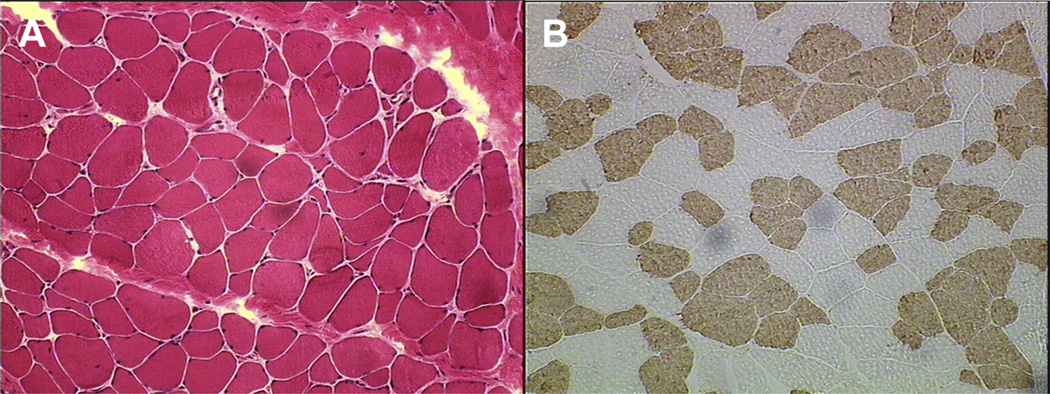
The histologic profile of muscle from patients with CP varies (Fig. 5A). Although the shape of individual muscle fibers may not change as drastically as it does in some other muscular disorders, most studies report a decrease in fiber size43,44 and an increase in variability of fiber size.43,45 Moderate rounding of muscle fibers has also been reported.44,45 In one study, capillary density was 30% lower in patients with CP.46 Lipid content has been shown to increase in some cases.43,44 Collagen content also appears to be increased,40,45 and this has been correlated with increased stiffness of muscle fiber bundle.40 Some investigators have even reported a significant correlation between collagen increases and patient function, using the Modified Ashworth Scale and patient balance measurements.45
Fig. 5.
Light micrographs of muscle from a patient with CP. (A) Hematoxylin and eosin permits evaluation of basic muscle tissue morphology. (B) Adenosine triphosphatase histochemical staining permits determination of muscle fiber type.
Myosin heavy chain, the major muscle contractile protein that determines fiber type, also changes in patients with CP, although the direction of these changes varies among muscles studied and techniques used (see Fig. 5B). One study, using nicotin-amide adenine dinucleotide (NADH) and adenosine triphosphatase (ATPase) staining, which indirectly reflect muscle oxidative capacity and contractile speed, respectively, reported increased fiber-size variability in patients with CP, and also found that patients with CP were more likely to have a strong predominance of one fiber type over the other (either type 1 or type 2). These differences were often greater than 40%, whereas typically developing patients showed no predominance.43 Another study using similar histochemical techniques in gastrocnemius biopsies reported increases in percentage of type 1 fiber and decreases in percentage of type 2 fiber, especially histochemically defined 2B fibers.47 In the adductor longus and triceps surea muscles, increased percentage of type 1 fiber was also reported with ATPase staining.44 A study of hamstring muscle in which myosin isoforms were electrophoretically separated demonstrated a 30% increase in type 1 myosin heavy chain in patients with CP. However, when monoclonal antibodies were used to label different myosin heavy chain isoforms in the biceps brachii, a dramatic increase in percentage of type 2 fiber was reported, with type 2X myosin heavy chain fibers making up 30% of CP muscle compared with 4% of typically developing muscle.46 Clearly variation exists among muscles, and further studies are needed to understand the impact of CP on predominance of muscle fiber type. From a physiologic perspective, however, percentage of fiber type is not likely to cause dramatic functional impairment, but rather reflects an altered use pattern of the muscle.
Gene Expression
Another technique used to understand muscle at the cellular level is gene expression analysis. Using microarrays, it is possible to compare thousands of genes simultaneously. Two recent studies quantified muscle genome-wide expression. The first, which compared muscles of the forearm, found distinct transcriptional differences between patients with and without CP.48 Alterations were seen in multiple pathways, with important differences in ECM-related genes, a myosin heavy chain fiber–type shift toward faster myosin, a decrease in oxidative metabolism, and altered genes that allow excitation-contraction coupling.48 The second study, which measured gene expression in hamstrings, found similar results, including a dramatic increase in ECM production– related gene expression (which matched the collagen content measured from the same samples) and decreased oxidative metabolism gene expression.49 These results agree with physiologic observations in patients with CP. These transcriptional data appear to reveal “confusion” in muscle cells that exist in the CP environment.
Stem Cells
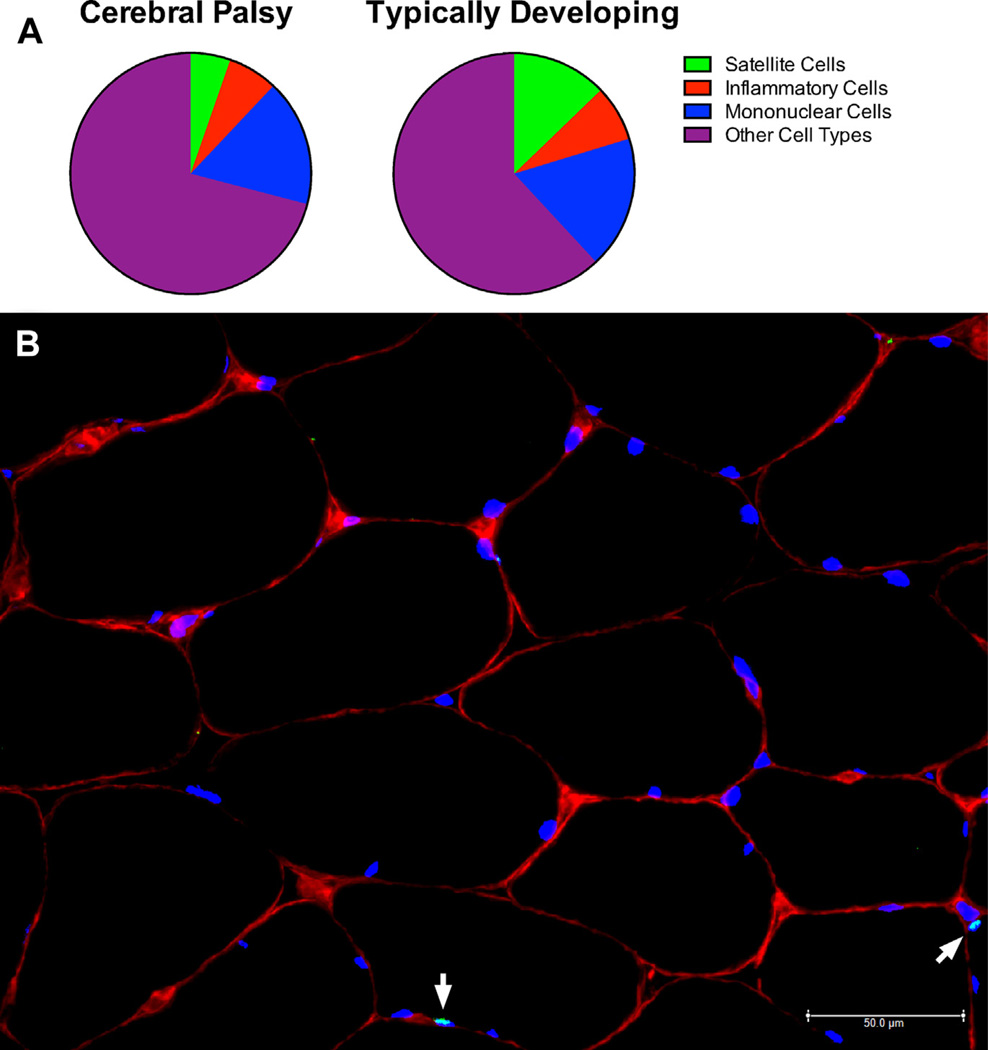
In a novel approach to understanding CP muscle, a recent publication highlighted the possible importance of certain muscle stem cells, called satellite cells, in patients with CP (Fig. 6). In one study, flow cytometry, a method used to count and isolate tagged cells of different types, was used to count cells in human biopsies.50 Although there was no change in either hematopoietic or endothelial cell numbers between groups, patients with CP had fewer than half as many satellite cells when compared with typically developing control subjects (see Fig. 6A). This observation reveals decreased Q9 intrinsic satellite cell number or satellite cell depletion in CP, and may indicate that this change contributes to abnormal sarcomere length, changes in material properties, and contracture formation. The question as to the cellular mechanism that could explain these changes remains unanswered. However, there is the exciting possibility that should this mechanism be determined, a new therapeutic approach to curing skeletal muscle contractures might be developed.
Fig. 6.
Fluorescence-activated cell sorting of several muscle mononuclear populations. (A) Whereas the percentage of other cell types such as mononuclear cells and inflammatory cells are unchanged in patients with CP, the fraction of satellite cells in CP muscle is half of what is found in TD muscle. (B) Immunohistochemical image of a satellite cell is shown in its native environment. Satellite cells are identified by looking for Pax7-positive staining (light green) on top of a cell nucleus (4′,6-diamidino-2-phenylindole [DAPI] positive; blue) that is located under the basal lamina (laminin staining; red). Satellite cells are indicated with white arrows. ([A] Data from Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol 2013;55(3):264–70; and [B] Courtesy of the National Skeletal Muscle Research Center, www.muscle.ucsd.edu; with permission.)
SUMMARY
Although CP is caused by a brain injury, critical symptoms manifest in the muscle. Contractures and spasticity seen clinically correspond to changes in muscle sarcomere length, fiber type, ECM concentration, fiber and fiber bundle stiffness, and even stem cell numbers. Better understanding of muscular changes and development of new treatments that focus on these aspects might lead to new avenues for improving function in patients with CP.
KEY POINTS.
Muscle from patients with cerebral palsy shows functional deficits such as decreased force production and range of motion.
Muscle is altered at a structural level, with decreased muscle body size, smaller-diameter fibers, and highly stretched sarcomeres (the force-producing unit of muscle).
Muscle from patients with cerebral palsy has altered extracellular matrix and connective tissue.
Decreased muscle stem cell numbers and altered gene expression have been reported in cerebral palsy.
Acknowledgments
Disclosure: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award numbers AR057393 and R24HD050837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Rosenbaum P, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;49:8–14. [PubMed] [Google Scholar]
- 2.Bax M, et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 3.Sankar C, Mundkur N. Cerebral palsy—definition, classification, etiology and early diagnosis. Indian J Pediatr. 2005;72(10):865–868. doi: 10.1007/BF02731117. [DOI] [PubMed] [Google Scholar]
- 4.Johnson A. Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44(09):633–640. [PubMed] [Google Scholar]
- 5.Arneson CL, et al. Prevalence of cerebral palsy: autism and developmental disabilities monitoring network, three sites, United States, 2004. Disabil Health J. 2009;2(1):45–48. doi: 10.1016/j.dhjo.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47(5):329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 7.Tilton AH. Approach to the rehabilitation of spasticity and neuromuscular disorders in children. Neurol Clin. 2003;21(4):853–881. doi: 10.1016/s0733-8619(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 8.Gorter JW, et al. Limb distribution, motor impairment, and functional classification of cerebral palsy. Dev Med Child Neurol. 2004;46(7):461–467. doi: 10.1017/s0012162204000763. [DOI] [PubMed] [Google Scholar]
- 9.Blix M. Die lange und die spannung des muskels. Skand Arch Physiol. 1894;5:149–206. [Google Scholar]
- 10.Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol. 1992;212(2):109–122. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- 11.Rowe RW. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell. 1981;13(4):681–690. doi: 10.1016/s0040-8166(81)80005-5. [DOI] [PubMed] [Google Scholar]
- 12.Järvinen TA, et al. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. J Muscle Res Cell Motil. 2002;23(3):245–254. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, et al. Micromechanical modeling of the epimysium of the skeletal muscles. J Biomech. 2008;41(1):1–10. doi: 10.1016/j.jbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Mauro A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9(2):493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206(3):451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- 16.Renault V, Thornell LE, Butler-Browne G, et al. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37(10–11):1229–1236. doi: 10.1016/s0531-5565(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 17.Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113(12):2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 18.Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- 19.Goldspink G. Sarcomere length during post-natal growth of mammalian muscle fibres. J Cell Sci. 1968;3(4):539–548. doi: 10.1242/jcs.3.4.539. [DOI] [PubMed] [Google Scholar]
- 20.Tabary JC, et al. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224(1):231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boakes JL, et al. Muscle adaptation by serial sarcomere addition 1 year after femoral lengthening. Clin Orthop Relat Res. 2007;456:250–253. doi: 10.1097/01.blo.0000246563.58091.af. http://dx.doi.org/10.1097/01.blo.0000246563.58091.af. [DOI] [PubMed] [Google Scholar]
- 22.Moreau NG, Falvo MJ, Damiano DL. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture. 2012;35(1):154–158. doi: 10.1016/j.gaitpost.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2009;51(10):800–806. doi: 10.1111/j.1469-8749.2009.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wren TA, et al. Achilles tendon length and medial gastrocnemius architecture in children with cerebral palsy and equinus gait. J Pediatr Orthop. 2010;30(5):479–484. doi: 10.1097/BPO.0b013e3181e00c80. [DOI] [PubMed] [Google Scholar]
- 25.Ballaz L, Plamondon S, Lemay M. Ankle range of motion is key to gait efficiency in adolescents with cerebral palsy. Clin Biomech. 2010;25(9):944–948. doi: 10.1016/j.clinbiomech.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Abel MF, et al. Muscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changes. J Pediatr Orthop. 1999;19(3):366–375. [PubMed] [Google Scholar]
- 27.Tammik K, et al. Quadriceps femoris muscle voluntary force and relaxation capacity in children with spastic diplegic cerebral palsy. Pediatr Exerc Sci. 2008;20(1):18–28. doi: 10.1123/pes.20.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA, Engsberg JR. Relationships between spasticity, strength, gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Arch Phys Med Rehabil. 2007;88(9):1114–1120. doi: 10.1016/j.apmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Barber L, Barrett R, Lichtwark G. Medial gastrocnemius muscle fascicle active torque-length and Achilles tendon properties in young adults with spastic cerebral palsy. J Biomech. 2012;45(15):2526–2530. doi: 10.1016/j.jbiomech.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Damiano DL, et al. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch Phys Med Rehabil. 2000;81(7):895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- 31.Barber L, Barrett R, Lichtwark G. Passive muscle mechanical properties of the medial gastrocnemius in young adults with spastic cerebral palsy. J Biomech. 2011;44(13):2496–2500. doi: 10.1016/j.jbiomech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Farmer SE, James M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil Rehabil. 2001;23(13):549–558. doi: 10.1080/09638280010029930. [DOI] [PubMed] [Google Scholar]
- 33.Legerlotz K, Smith HK, Hing WA. Variation and reliability of ultrasonographic quantification of the architecture of the medial gastrocnemius muscle in young children. Clin Physiol Funct Imaging. 2010;30(3):198–205. doi: 10.1111/j.1475-097X.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 34.Mohagheghi AA, et al. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech. 2007;22(6):718–724. doi: 10.1016/j.clinbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Malaiya R, et al. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol. 2007;17(6):657–663. doi: 10.1016/j.jelekin.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Mohagheghi AA, et al. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol. 2008;50(1):44–50. doi: 10.1111/j.1469-8749.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 37.Barber LE, et al. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev Med Child Neurol. 2011;53(6):543–548. doi: 10.1111/j.1469-8749.2011.03913.x. [DOI] [PubMed] [Google Scholar]
- 38.Smeulders MJ, et al. Overstretching of sarcomeres may not cause cerebral palsy muscle contracture. J Orthop Res. 2004;22(6):1331–1335. doi: 10.1016/j.orthres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Lieber RL, Fridén J. Spasticity causes a fundamental rearrangement of muscle–joint interaction. Muscle Nerve. 2002;25(2):265–270. doi: 10.1002/mus.10036. [DOI] [PubMed] [Google Scholar]
- 40.Smith LR, et al. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589(10):2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27(2):157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 42.Lieber RL, et al. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28(4):464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 43.Rose J, et al. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12(6):758–768. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- 44.Marbini A, et al. Immunohistochemical study of muscle biopsy in children with cerebral palsy. Brain Dev. 2002;24(2):63–66. doi: 10.1016/s0387-7604(01)00394-1. [DOI] [PubMed] [Google Scholar]
- 45.Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001;43(5):314–320. doi: 10.1017/s0012162201000597. [DOI] [PubMed] [Google Scholar]
- 46.Pontén EM, Stal PS. Decreased capillarization and a shift to fast myosin heavy chain IIx in the biceps brachii muscle from young adults with spastic paresis. J Neurol Sci. 2007;253(1–2):25–33. doi: 10.1016/j.jns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Ito J, et al. Muscle histopathology in spastic cerebral palsy. Brain Dev. 1996;18(4):299–303. doi: 10.1016/0387-7604(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 48.Smith L, et al. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics. 2009;2(1):44. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith LR, et al. Transcriptional abnormalities of hamstring muscle contractures in children with cerebral palsy. PLoS One. 2012;7(8):e40686. doi: 10.1371/journal.pone.0040686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol. 2013;55(3):264–270. doi: 10.1111/dmcn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]