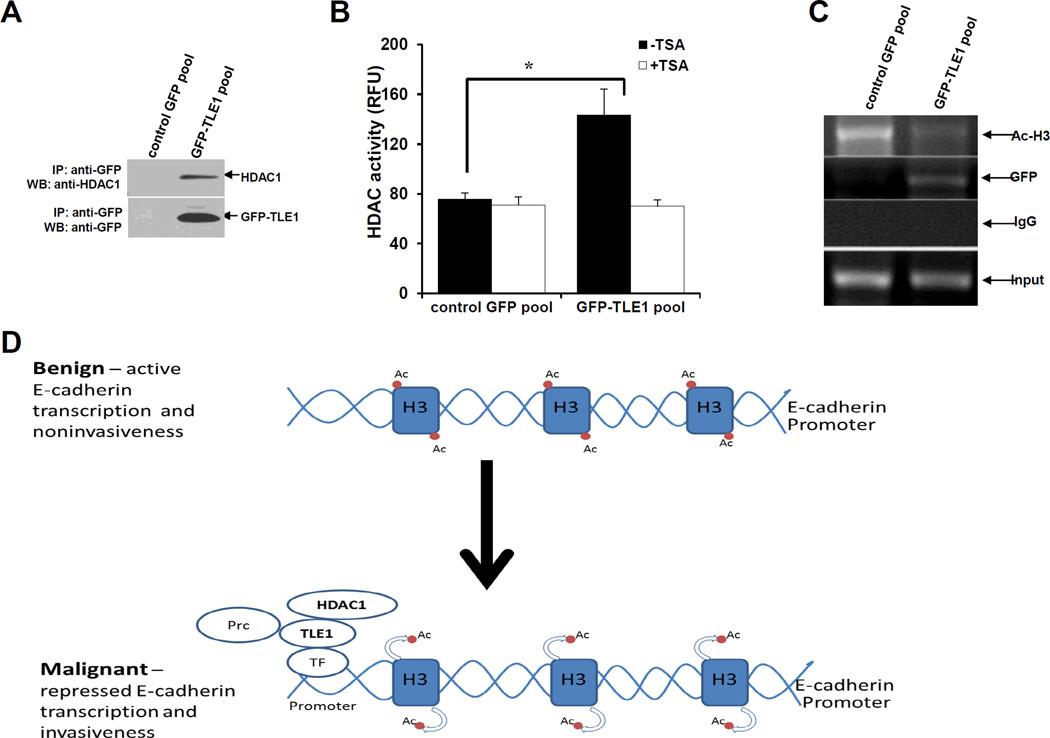
Fig. 4.
TLE1 interacts with HDAC1 and recruits HDAC activity to the endogenous E-cadherin promoter. A. Control GFP and GFP-TLE1 cells were harvested and cell extracts were prepared, immunoprecipitated (IP) with agarose-immobilized anti-GFP, and immunoblotted with anti-HDAC1 and anti-GFP antibodies. B. Control GFP and GFP-TLE1 cells treated or untreated with 300 nM TSA for 24h were subjected to immunoprecipitation (IP) with anti-GFP antibodies, and the HDAC activity in the anti-GFP immunoprecipitated fractions was determined as described in materials and methods. C. Control GFP and GFP-TLE1 cells were analysed by ChIP assays with anti-GFP, anti-acetyl-histone H3 (Ac-H3), and control IgG as detailed in the materials and methods. D. A model depicting the mechanism of TLE1-induced E-cadherin repression. In benign cells wherein active E-cadherin transcription is occurring, the E-cadherin promoter is not occupied by TLE1 and exhibits basal Histone 3 acetylation. In aggressive lung cancer, the increased expression of TLE1 will lead to its binding to the promoter of E-cadherin (likely through DNA binding transcriptional regulatory factors (TF)) followed by recruitment of HDAC1, deacetylation of Histone H3, and repression of E-cadherin transcription resulting to increased invasiveness. The inability of HDAC inhibitors to completely block TLE1-mediated E-cadherin suppression indicates TLE1 may also recruit additional chromatin remodelling proteins such as the histone methylating Polycomb repressive complex (Prc) (6). In B, three independent experiments were performed in triplicates, * indicates p<0.05 by Student’s t test.