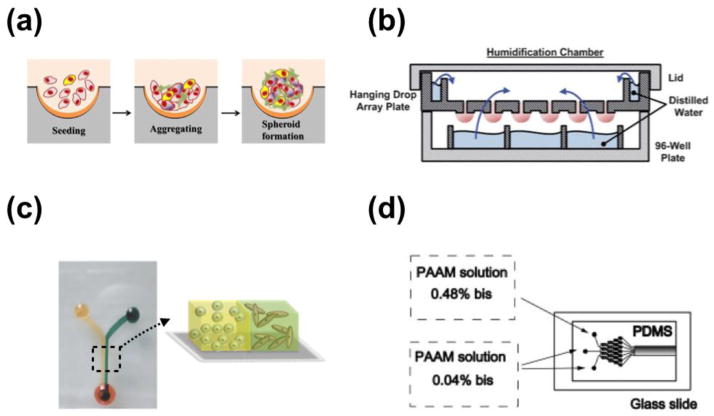
Fig. 2.
Representative microfluidic 3D systems. (a) schematic illustration of tumor spheroids generated in microfabricated well. Reproduced from Ref. [34] with permission. (b) Illustration of he hanging drop array plate to culture 3D spheroids. The 384 hanging drop array plate is sandwiched between a 96-well plate filled with distilled water and a standard-sized plate lid. Distilled water from the bottom 96-well plate and the peripheral water reservoir prevent serious evaporation of the small volume hanging drops. Reproduced from Ref. [36] with permission. (c) 3D micro-compartmentalization driven by laminar flow in microchannels. Reproduced from Ref. [40] with permission. Drops of cell containing polymer solutions are loaded onto the inlet ports. Laminar flow leads to two side-by-side 3D compartments. (d) Schematic diagram of the microfluidic gradient device used for photopolymerization of hydrogels. Reproduced from Ref. [101] with permission. the device consists of a pat- terned PDMS mold attached to an activated glass slide. All of the inlets are filled with a solution containing 8 % acrylamide and the photoinitiator. To generate a gradient in the crosslinker concentration, 0.04 % bis was added to two adjacent inlets, and 0.48 % bis to the third inlet.