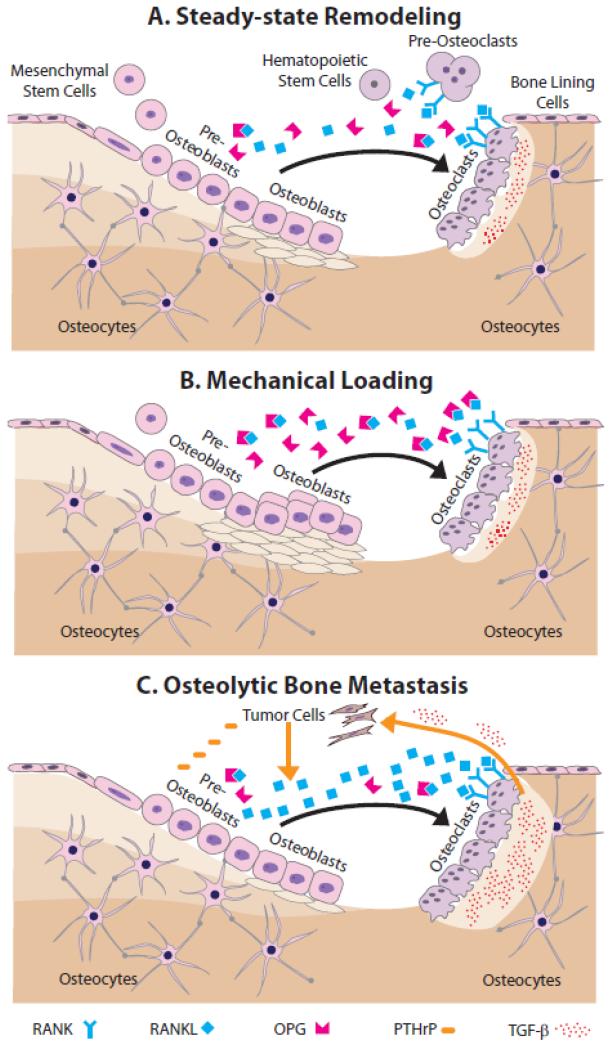
Figure 4. Steady-state remodeling and the effects of mechanical loading and bone metastasis.
A) During steady-state remodeling, mesenchymal stem cell-derived pre-osteoblasts recruit hematopoietic stem cells and induce their differentiation via RANK-RANKL signaling into large, multinucleated osteoclasts. RANK-RANKL signaling is modulated by osteoblastic secretion of OPG, which is a decoy receptor for RANKL. Mature osteoclasts remove matrix, and then apoptose. Next, active osteoblasts secrete new bone matrix, which subsequently mineralizes. After this, osteoblasts become quiescent bone lining cells, undergo apoptosis, or terminally differentiate into osteocytes. B) During mechanical loading, more mesenchymal precursors commit to the osteoblastic lineage, their differentiation and matrix deposition is enhanced, and apoptosis is inhibited. C) During breast cancer bone metastasis, tumor cells secrete a variety of osteolytic factors including PTHrP, which increases osteoblastic secretion of RANKL thus leading to greater osteoclastogenesis. Elevated resorption of the bone matrix, in turn, releases more pro-tumorigenic growth factors, most notably TGF-β, that further stimulates tumor growth.