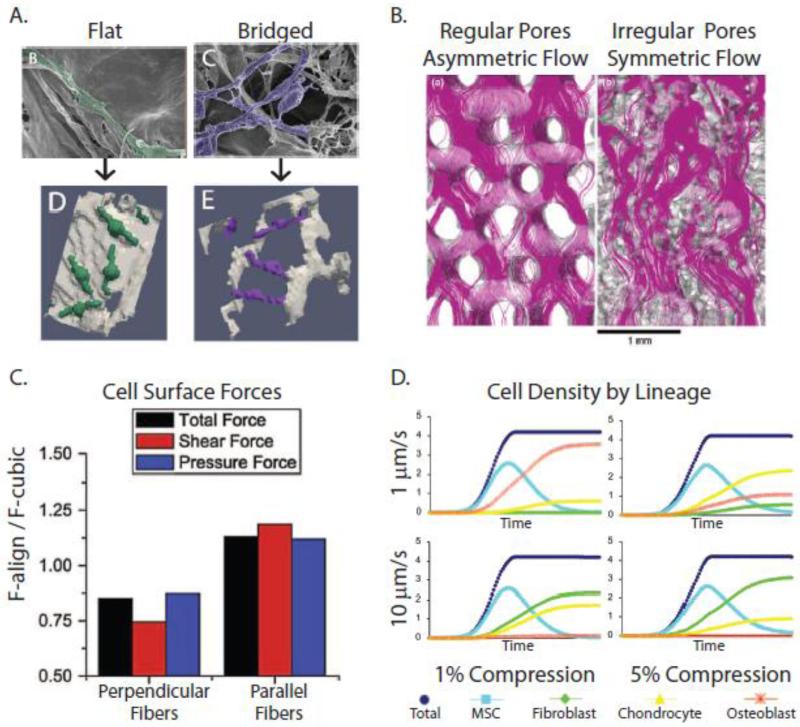
Figure 8. Computational simulations of loading-induced changes of cell behavior in 3-D porous scaffolds.
A) Computational models of MC3T3 pre-osteoblasts within collagen-glycosaminoglycan (CG) scaffolds (Bottom) rendered from high-resolution scanning electron microscopy (Top) revealed that cell detachment is proportional to flow rate and inversely proportional to pore size [234]. B) Flow patterns through porous scaffolds depend on pore orientation. In polycaprolactone (PCL) scaffolds, pores are more regularly-oriented, resulting in more asymmetric flow and shear stresses. In contrast, in silk fibroin scaffolds, the pores are more irregularly-oriented, ‘conditioning’ the flow, and shear stresses are more uniform [238]. C) Perpendicular alignment of ECM fibers results in lower cellular strains (stress-shielding), while parallel alignment of the fibers increases shear stresses on cells, as demonstrated using computational fluid dynamics simulations [231]. D) When FE and CDF approaches were combined to model the effect of specific combinations of compression and perfusion on MSCs cultured within CG scaffolds, differing patterns of MSC differentiation into fibroblasts, chrondrocytes, or osteoblasts were predicted [233]. Reproduced with permission from John Wiley and Sons, Springer, and Elsevier.