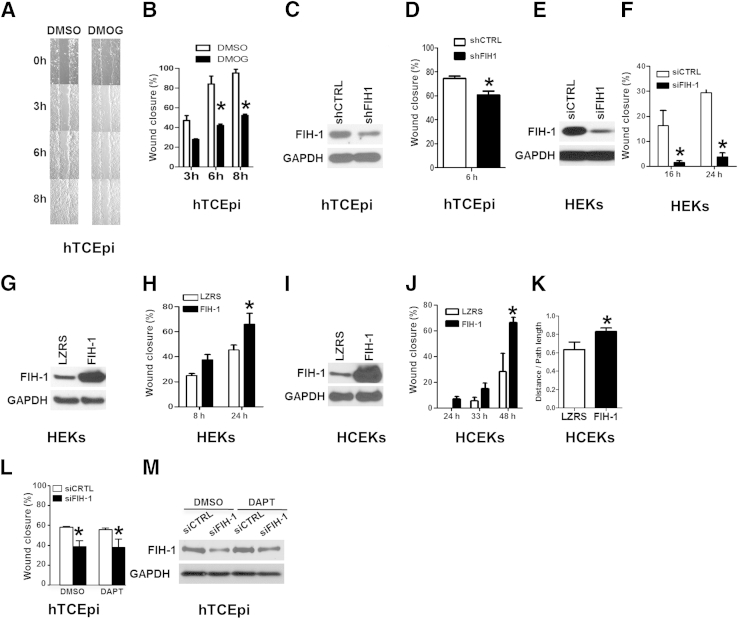
Figure 2.
FIH-1 positively regulates keratinocyte cell migration. A and B: The limbal-derived corneal epithelial hTCEpi cells were treated with the cell-permeable FIH inhibitor dimethyloxalylglycine (DMOG) or dimethyl sulfoxide (DMSO) for 2 hours. At this time, confluent hTCEpi cells were scratch-wounded and then allowed to migrate until wounds were closed (8 hours; vehicle). C and D: hTCEpi cells were lentivirally transduced with an shRNA against either FIH-1 (shFIH-1) or sh control (shCTRL). These transduced cells were used for immunoblot analysis of FIH-1 and GAPDH (C) and scratch-wound–healing assays (D). E and F: HEKs were transfected with an siRNA pool against either FIH-1 (siFIH-1) or scrambled control (siCTRL) and then used for immunoblot analysis of FIH-1 and GAPDH (E) and scratch-wound–healing assays (F). G–J: HEKs and HCEKs were retrovirally transduced with FIH-1 or empty vector (LZRS) and then immunoblot analyses of FIH-1 and GAPDH were performed in HEKs (G) and HCEKs (I) and scratch-wound–healing assays were conducted in HEKs (H) and HCEKs (J). The percentage of wound closure from a representative experiment was measured temporally using AxioVision software. K: Single-cell migration assays were performed in HCEKs transduced with either FIH-1 or empty vector (LZRS) using a Nikon Biostation system. The persistency of migration path was calculated based on the ratios of the distance between the starting and ending points and the total path length. L: Scratch-wound assays were performed on hTCEpi cells transfected with either siFIH-1 or siCTRL after 16-hour pretreatment with 10-μmol/L DAPT. DAPT is a γ-secretase inhibitor that blocks Notch signaling. M: Immunoblot analyses of FIH-1 and GAPDH were performed after scratch-wounding. Data are means ± SD (B, D, F, H, J, K, and L). n = 3 (B, D, F, H, J, and L); n = 8 (K). ∗P < 0.05.