Fig. 6.
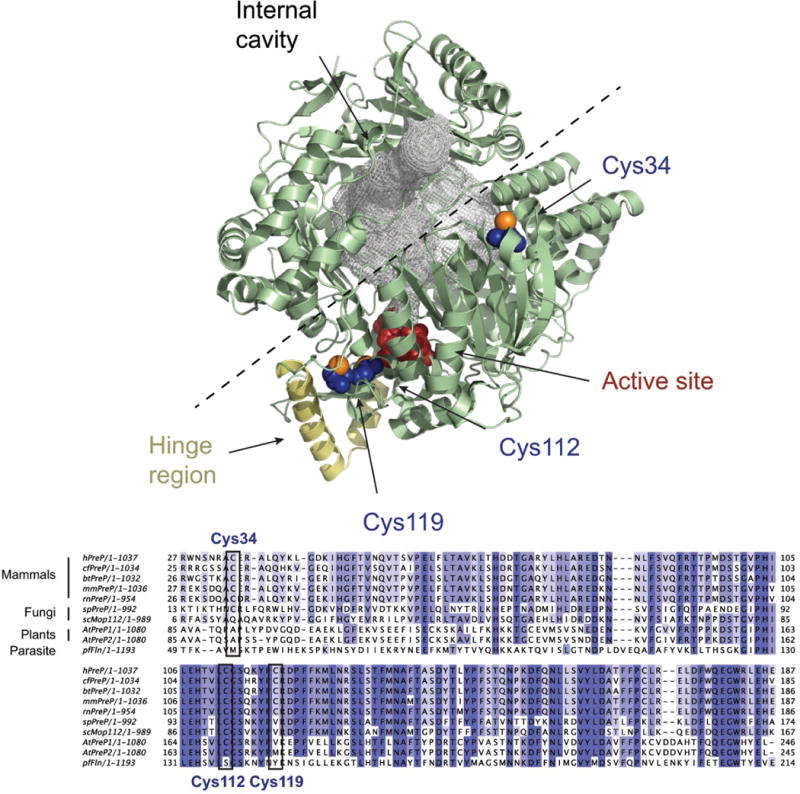
Crystal structure of hPreP. Upper panel. Overview of the hPreP structure [27] (4L3T, pale green) with the residues composing the active site shown in red and the hinge region colored in yellow. The internal cavity in hPreP is filled with a gray mesh, and the dashed line shows the proposed path of opening. The oxidation-susceptible residues Cys34, Cys112, and Cys119 are shown in blue, except for the sulfur atoms which are orange. The figure was prepared using PyMOL [30].
Lower panel. Alignment of PreP sequences from Homo sapiens (hPreP), Canis familiaris (cfPreP), Bos taurus (btPreP), Mus musculus (mmPreP), Rattus norvergicus (rnPreP), Schizosaccharomyces pombe (spPreP), S. cerevisiae (scMop112), A. thaliana (AtPreP1 and AtPreP2) and Plasmodium falciparum (pfFln). The alignment was generated with Clustal Omega [31] and is restricted to the region around Cys34 to Cys119.
