Figure 1.
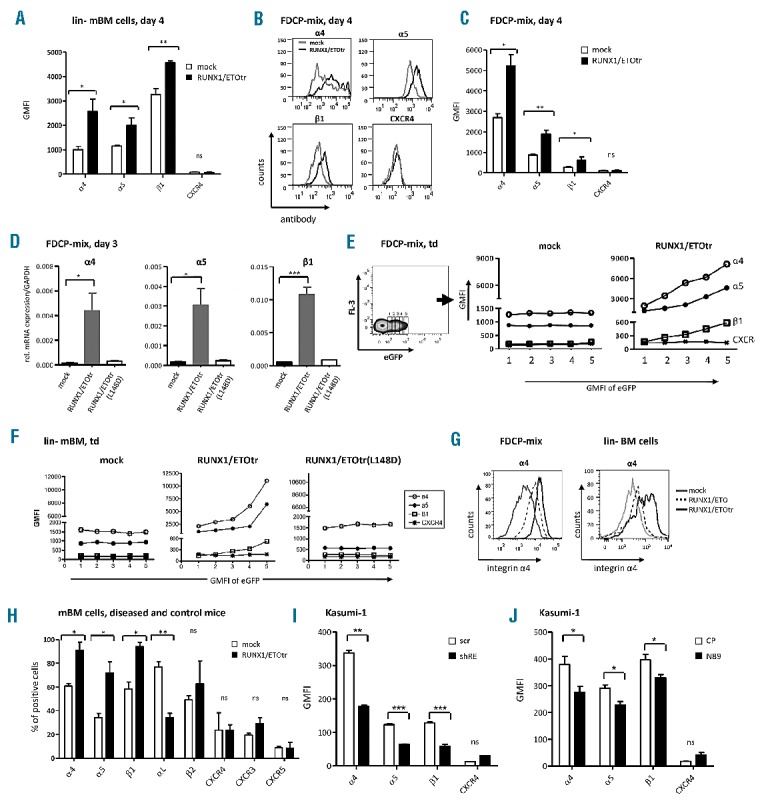
RUNX1/ETO up-regulates integrin α4, α5 and β1 subunit expression in hematopoietic progenitor cells. (A) lin- mBM cells were transduced with RUNX1/ETOtr and mock control lentiviral vectors and measured for integrin α4, α5 and β1 subunit and CXCR4 expression using flow cytometry on Day 4 after transduction. (B) Integrin subunit expression in transduced FDCP-mix cells at Day 4 after transduction. (C) Quantitative analysis of (B). (D) mRNA expression of the integrin α4, α5 and β1 subunits amplified using qPCR by the corresponding integrin primers (Applied Biosystems, Darmstadt, Germany) and normalized to GAPDH. Total RNA from FDCP-mix cells was isolated using an RNeasy micro kit (Qiagen, Hilden, Germany) on Day 3 after transduction. Next, 500 ng of RNA was reverse transcribed into cDNA using superscript III reverse transcriptase (Invitrogen, Darmstadt, Germany). (E and F) Representative examples of integrin α4, α5 and β1 subunit expression levels in transduced FDCP-mix and lin- mBM cells shown for different eGFP expression levels. (G) Integrin α4 subunit cell surface expression of FDCP-mix and lin- BM cells four days after transduction. (H) Analysis of adhesion molecule expression in bone marrow of mock- and RUNX1/ETOtr-lin- mBM cells transplanted mice. (I) Expression levels of α4, α5 and β1 integrin subunits and CXCR4 in t(8;21) positive Kasumi-1 cells transduced with lentiviral vectors expressing scramble control (scr) or shRNA targeting the breakpoint region of RUNX1/ETO (shRE) as measured by FACS. (J) Expression of α4, α5 and β1 integrin subunits and CXCR4 in Kasumi-1 cells transduced with lentiviral vectors expressing control peptides (CP) or RUNX1/ETO inhibitory peptides (N89) as measured by FACS. n=3; mean±SD. *P<0.05; **P<0.01; ***P<0.001; ns: not significant. GMFI: geometrical mean fluorescence intensity.
