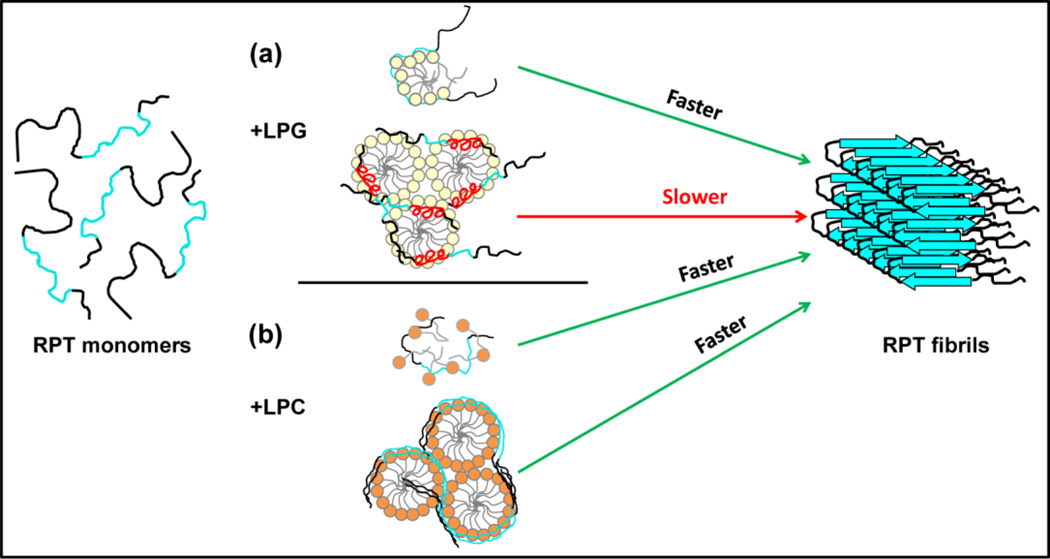
Fig. 7.
Proposed mechanism for lysolipid-modulated RPT aggregation. RPT molecules are depicted with the amyloidogenic region colored cyan. (a) RPT aggregation in the presence of LPG. LPG monomers (top) stimulate RPT aggregation by specifically binding to the C-terminus, forming protein-lipid micelle-like structures likely through hydrogen bonding, and promoting intermolecular interaction between RPT molecules. In contrast, LPG micelles (bottom) induce RPT to develop α-helices (red) possibly within the amyloid core region (repeats 8–9), preventing β-sheet formation and inhibiting aggregation. (b) RPT aggregation in the presence of LPC. The non-specific binding of LPC monomers (top) exposes the hydrophobic regions of RPT, which facilitates protein-protein association, leading to faster aggregation. The unfolded RPT molecules accumulate on the surface of LPC micelles (bottom), functioning as a protein-concentrating platform and enhancing aggregation kinetics.