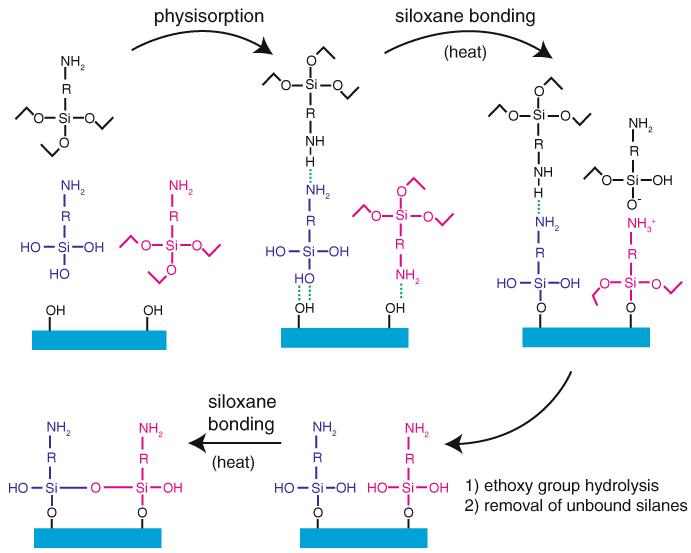
Fig. 4.
Aminopropyltriethoxysilane functionalization of glass using APTES (R=–(CH2)3–) or AEAPTES (R=–(CH2)3–NH(CH2)2–). An appropriately cleaned glass surface containing a high density of silanol groups is exposed to the aminosilane in nearly anhydrous acetone, yielding a diverse mixture of free silanes and silanols in solution, which then physisorb onto the surface via hydrogen bonding and/or ionic interactions (several configurations in addition to the ones shown are possible). The addition of heat drives the formation of siloxane bonds between the physisorbed silanes/silanols and the glass surface by supplying energy and removing condensation products (water and ethanol) by evaporation. This reaction is catalyzed by the terminal amine group. Rinsing in ethanol and water removes any remaining physisorbed aminosilane deposits and leads to hydrolysis of remaining ethoxy groups on the bound silanes, converting them to silanols. A final heating/drying helps these silanol groups form intramolecular siloxane linkages (siloxane bonds may also form via reaction of adjacent ethoxy and silanol groups, as during the initial bonding to the surface). This conceptual scheme does not illustrate several concurrent pathways that also lead to stable binding of aminosilanes to the glass surface (e.g., aminosilane oligomerization in solution, followed by physisorption and binding to the surface). The final product of this treatment is a glass surface densely covered in covalently attached amines