Abstract
Unripe fruit of Annona muricata Linn. (Annonaceae) (soursop) is used in traditional African medicine for the treatment of neuralgia, rheumatism, and arthritic pain. This study sought to investigate the analgesic and anti-inflammatory effects of lyophilized fruit extract of Annona muricata (AM) in rodents. The analgesic activity was evaluated using the mouse writhing, formalin, and hot-plate tests while the anti-inflammatory action was investigated using the carrageenan-induced rat paw edema and xylene-induced ear edema tests. Pretreatment with AM (50, 100, and 200 mg/kg, p.o.) produced dose-dependent (P<.001) inhibition of writhes and formalin-induced pain in the late phase. AM and morphine produced time-course increase in pain threshold in hot-plate test. However, the analgesic effect elicited by AM was reversed (P<.05) by naloxone pretreatment. Similarly, the time-dependent increase in paw circumference induced by carrageenan was inhibited by AM treatment with peak effect (0.23±0.10 cm; P<.001, 200 mg/kg; 6 h), which was comparatively similar to that of diclofenac treated. Further, the xylene-induced ear edema was significantly reduced by AM (50 or 100 mg/kg) pretreatment; however, the anti-inflammatory effect elicited by AM was prevented by pretreatment of mice with NG-nitro-l-arginine (20 mg/kg, i.p., nitric-oxide synthase inhibitor) 15 min before AM (200 mg/kg, p.o.). The in vitro cyclooxygenase assay also showed that AM produced concentration-dependent inhibition of both cyclooxygenase (COX)-1 and COX-2 activity by 39.44%±0.05% and 55.71%±0.12%, respectively, at 100 μg/mL. In conclusion, A. muricata possesses analgesic effect through interaction with opioidergic pathway and anti-inflammatory property through inhibition of chemical mediators of inflammation.
Key Words: : carrageenan, cyclooxygenase, formalin test, inflammation, naloxone, nitric oxide
Introduction
Annona muricata Linn. (Annonaceae) (soursop, guanabana, graviola, or corossol), “Eko oyinbo” or “Eko omode” (Yoruba; Southwest, Nigeria), is cultivated throughout the tropical regions of the world.1 The ripe fruits are highly perishable, as they become soft and are easily bruised. The fruits have a unique pleasant, subacid, and aromatic flavor, but in their fresh form they are not as popular as other tropical fruits.1 Moreover, soursop pulp has been processed into a nectar and its quality evaluated.2
Fruits of A. muricata are taken internally for worms, fever, to increase mother's milk after child birth, and as an astringent for diarrhea and dysentery3; unripe fruit mixed with olive oil was used for neuralgia, rheumatism, and arthritic pain.4 The leaves are used in traditional medicine to treat headaches, hypertension, cough, and asthma and used as antispasmodic, sedative, and nervine for heart condition.5,6 Annonaceous acetogenins, from Annona muricata L., were found to be a promising new antitumor and anticancer agent in numerous in vitro studies.7 These acetogenins were demonstrated to be selectively toxic against various types of the cancerous cells without harming healthy cells.8 Various other plants from this family have also been reported for their cytotoxic potential.9,10 Despite the use of the unripe fruit of A. muricata in the management of painful conditions and inflammatory disorders, no scientific investigation has been carried out to ascertain the claimed folklore uses in these conditions. Hence, this study was carried out to investigate the analgesic and anti-inflammatory properties of the lyophilized fruit extract of A. muricata in rodents and its possible mechanism of actions.
Materials and Methods
The fruits of A. muricata were obtained from Mushin market, Mushin, Lagos state, Nigeria. It was identified and authenticated by Mr. T.K. Odewo, a forestry expert in the Department of Botany Herbarium, Faculty of Science, University of Lagos, Akoka, Lagos, Nigeria, and a voucher specimen (LUH 5081) was deposited in the Herbarium of the Department.
Extraction
The pericarps of unripe fruits (1500 g) were removed with the seeds. The juice was extracted using a juice extractor (Nikashi, Japan). The juicy exudate was freeze-dried using a vacuum freeze drier (Telstar Model 9955-12, Grade 371H, United Kingdom) and was stored until ready to use. The percentage yield was 23.50%.
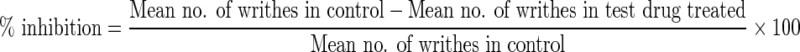 |
Drugs and chemicals
Morphine sulfate (Martindale Pharmaceuticals, Ranford, United Kingdom), diclofenac (Hovid Pharmaceuticals, Perak, Malaysia), glibenclamide (Swiss Pharma Nig. Limited, Agege, Nigeria), naloxone (Hameln Pharma Plus GMBH, Hameln, Germany), formalin, carrageenan, xylene, NG-nitro-l-arginine, glacial acetic acid, napthylethylenediamine dihydrochloride (NED), phosphate-buffered saline, sodium nitroprusside (SNP), sulfanilamide (Sigma Aldrich, St. Louis, MO, USA), and colorimetric cyclooxygenase (COX; ovine) inhibitor screening assay kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Laboratory animals
Male Sprague–Dawley rats (120–130 g) and 8-week-old Swiss albino mice (20–25 g) were obtained from the Laboratory Animal Centre, College of Medicine, University of Lagos, Lagos, Nigeria. The animals were kept under standard environmental conditions (23–25°C, 12 h/12 h light/dark cycle) and fed with standard rodent pellet (Livestock Feed PLC, Lagos, Nigeria), and tap water was available ad libitum but food was not allowed 12 h prior to and until completion of the experiment. Experiments were performed according to international ethical standards approved by the Research Grant and Animal Experimentation Committee of the College of Medicine, University of Lagos, Lagos, Nigeria, and were in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.11
Acute toxicity study
Mice were generally fasted for 12 h before the test. Five groups of mice (n=6) received 10 mL/kg distilled water and A. muricata (250, 500, 1000, and 4000 mg/kg, p.o.). Mice were observed for toxic symptoms and behavioral changes (sedation, hyperactivity, diarrheal, writhing, piloerection, restlessness, etc.) for 2 h postadministration and 14 days for signs of delayed toxicity.12
Antinociceptive test
Acetic-acid-induced abdominal constriction test
Male albino mice (20–25 g, n=6) were treated with vehicle (distilled water, 10 mL/kg), A. muricata (50, 100, or 200 mg/kg, p.o.), or diclofenac (20 mg/kg, p.o.),13 60 min prior to intraperitoneal injection of 0.6% (v/v) acetic acid (10 mL/kg) to induce writhes (i.e., contraction of the abdominal muscle with simultaneous stretching of the hind limbs), as described by Koster et al.14 The number of writhes was counted cumulatively over a period of 20 min by an observer unaware of the treatment groups. The percentage inhibition of writhing reflex was calculated using the formula:
Formalin test
Mice fasted overnight were divided into five groups (n=6). The different groups of animals were treated with distilled water (10 mL/kg, p.o.), A. muricata (50, 100, or 200 mg/kg, p.o.), or morphine (10 mg/kg, s.c.).13 One hour after drug administration or 30 min after subcutaneous injection, 20 μL of formalin (1% v/v in saline) was injected into the right hind paw of each mouse. The time (in sec) spent in licking or biting the injected paw, indicative of pain, was recorded for each animal. The responses of the mice were observed for the first 5 min (neurogenic phase) and 15–30 min (inflammatory phase) postformalin injection13 by an observer unaware of the treatment groups.
Hot-plate test (central analgesic activity)
To investigate the involvement of central mechanism in the analgesic effect elicited by A. muricata, the hot-plate assay was carried out according to the method described by Eddy and Leimbach.15 Male albino mice (20–25 g, n=6) were placed on Columbus analgesiometer maintained at 55°C±1°C. Response time was recorded as the time elapsed before the mouse responded by licking, flicking of a hind paw, or jumping. Only mice with a control response of 2–4 sec were included in the study. Each mouse acted as its own control. Prior to treatment, the reaction time of each mouse was carried out at 0- and 15-min intervals. The average of the two readings was taken as the initial reaction time. Animals were treated with distilled water (10 mL/kg, p.o.), A. muricata (50, 100, or 200 mg/kg, p.o.), or morphine (10 mg/kg, s.c.).13 The nociceptive response was measured 30 min post-treatment and every 30 min for 2 h. The increase in latency time in relation to the initial time for each group was taken as an index of analgesic activity. Ten-second post-treatment cutoff time was used to prevent tissue damage. Percentage of the maximum possible effect (% MPE) was calculated using the formula:
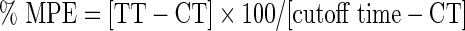 |
TT=mean reaction time to pain in drug, CT=mean reaction time to pain threshold in control.
Elucidation of mechanism(s) of analgesic activity
Involvement of opioid pathway
The involvement of opioidergic pathway in the analgesic action produced by A. muricata was investigated through subcutaneous injection of naloxone (5 mg/kg, nonselective opioid receptor antagonist)16 15 min before A. muricata (200 mg/kg, p.o.) and, after 1 h, formalin (20 μL of 1% solution) was injected into the right hind paw. The time (in sec) spent in licking or biting the injected paw, indicative of pain, was recorded for each animal. The responses of the mice were observed for the first 5 min (neurogenic phase) and 15–30 min (inflammatory phase) postformalin injection13 by an observer unaware of the treatment groups.
Involvement of ATP-sensitive potassium channels
The role played by potassium channels in the analgesic effect of A. muricata was evaluated through pretreatment of mice with glibenclamide (10 mg/kg i.p., an ATP-sensitive potassium channel inhibitor),17 and after 15 min they received A. muricata (200 mg/kg, p.o.) and, 1 h after extract administration, 20 μL of formalin (1% in normal saline) was injected into the right hind paw. The duration of paw licking was observed for early (0–5 min) and late phases (15–30 min), respectively.18 The antagonists were used at doses effective in blocking the in vivo effects induced by opioid and potassium channel receptor agonists in mice.16,17
Carrageenan-induced rat paw edema
Male Sprague–Dawley rats (120–130 g) were randomly divided into groups of six animals each, and were used after a 12-h fast but allowed free access to water except during the experiment. Edema was induced by injection of 100 μL of carrageenan (1% in normal saline) into the plantar surface of the right hind paw.19 The animals were treated with vehicle (distilled water, 10 mL/kg), A. muricata (50, 100, or 200 mg/kg, p.o.), or diclofenac (20 mg/kg, p.o.),13 1 h before injection of carrageenan. Paw diameter was measured using the cotton thread method of Bamgbose and Noamesi,20 before and 1, 2, 3, 4, 5, and 6 h after injection of carrageenan. Anti-inflammatory activity was expressed as the percentage reduction in edema in treated rats in comparison to controls.21
Xylene-induced ear edema
Mice were allotted to five groups (n=6). Thirty minutes after oral treatment of mice with distilled water (10 mL/kg), prednisolone [(20 mg/kg),22 steroidal anti-inflammatory drug], or A. muricata (50, 100, or 200 mg/kg), edema was induced in each mouse by instilling 30 μL of xylene to the inner surface of the right ear. Fifteen minutes after xylene application, the animals were euthanized under ether anesthesia and both ears were cut off, sized, and weighed. The mean of the difference between the right and left ears was determined for each group and percentage inhibition was calculated.22,23
Involvement of nitric oxide pathway in the anti-inflammatory effect of A. muricata
Male Sprague–Dawley rats (120–130 g) were pretreated with NG-nitro-l-arginine (L-NNA; 20 mg/kg, i.p., nitric-oxide synthase inhibitor)24; 15 min after pretreatment, A. muricata (200 mg/kg, p.o.) was administered and, 1 h post-treatment, 100 μL of carrageenan (1% w/v in normal saline) was injected into the right hind paw. Paw diameter was measured using the method of Bamgbose and Noamesi,20 before and 1, 2, 3, 4, 5, and 6 h after injection of carrageenan. Anti-inflammatory activity was expressed as the percentage reduction in edema.14
Colorimetric COX inhibitory assay
The effect of graded concentration of AM on COX activity was evaluated using the colorimetric COX (ovine) inhibitor screening assay kit (No. 760111; Cayman). The peroxidase activity was assayed by monitoring the appearance of oxidized N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) at 590 nm using plate reader as instructed by the manufacturer. Aspirin and celecoxib served as standard reference drugs. The standard reference drugs and AM were dissolved in 1% v/v dimethylsulfoxide (DMSO; 12.5, 50, 100, and 200 μg/mL). The plate was shaken for 30 sec and incubated for 5 min at 25°C and then 20 μL of the colorimetric substrate solution (TMPD) was added to all the wells. Twenty microliters of arachidonic acid was also added to all the wells. The plate was shaken for a few seconds and incubated for 5 min at 25°C. The absorbance was read at 590 nm using a microplate reader (n=3). The mean absorbance for each background, sample, and inhibitor treated was determined. The percentage of COX activity inhibition was calculated using the formula:
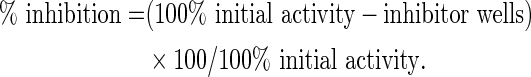 |
Nitric oxide radical scavenging
At physiological pH, nitric oxide generated from aqueous SNP solution interacts with oxygen to produce nitrite ions, which may be quantified by the Griess Illosvoy reaction.25 The reaction mixture contained 10 mM SNP, phosphate-buffered saline (pH 7.4), and graded doses of AM (0–100 μg/mL) in a final volume of 3 mL. After incubation for 150 min at 25°C, 1 mL of sulfanilamide (0.33% in 20% glacial acetic acid) was added to 0.5 mL of the incubated solution and allowed to stand for 5 min. Then, 1 mL of NED (0.1% w/v) was added and the mixture was incubated for 30 min at 25°C. The pink chromophore generated during diazotization of nitrite ions with sulfanilamide and subsequent coupling with NED was measured spectrophotometrically at 540 nm against a blank sample. All tests were performed six times. Ascorbic acid was used as a standard.
Statistical analysis
Results obtained were expressed as mean±SEM. The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc multiple-comparison test for antinociceptive study while two-way ANOVA followed by Bonferroni post hoc multiple-comparison test was used for anti-inflammatory study. Values were considered significant when P<.05.
Results and Discussion
Findings from this study showed that the lyophilized fruit extract of A. muricata possesses analgesic and anti-inflammatory effects possibly mediated via interaction with opioidergic pathway and inhibition of chemical mediators of inflammation. The analgesic properties of the fruit extract were investigated using both chemical and thermal models of nociception in mice. The fruit extract of A. muricata (50–200 mg/kg) protected mice against both chemical- and thermal-induced noxious stimuli, which were evidenced from the acetic-acid-induced writhing, formalin, and hot-plate tests. Acetic-acid-induced mouse writhing test is considered to be a very sensitive model since it can detect antinociceptive effect of test drugs at the lowest dosages that might not be effective in hot-plate or tail-flick test due to direct interaction of the extracts/compounds with the various peripheral receptors within the peritoneal cavity.26 However, this test also has been regarded as a nonspecific test as it could not be used to stipulate the involvement of peripheral or central mechanism in the analgesic effect of A. muricata.27 As shown in Table 1, oral administration of A. muricata (50–200 mg/kg) produced dose-dependent and significant (P<.01) inhibition of acetic-acid-induced abdominal constrictions in mice, with maximum inhibition of 55.19% at 200 mg/kg while diclofenac treatment produced 60.38% inhibition of writhes. Due to poor specificity of this model (e.g., muscle relaxant can also reduce number of writhes), and to differentiate between the central and peripheral analgesic effects of AM, the formalin test was carried out. This model simulates clinical symptoms of pain,28 which involves two distinct phases. The first phase, neurogenic pain, occurs 0–5 min after the injection of formalin.29 Then, after a quiescent period, a second phase, inflammatory pain, occurs 15–30 min postformalin injection.29 The first phase results from direct stimulation of nociceptors.18 Moreover, the injection of formalin into the right hind paw causes an immediate and intense increase in the spontaneous activity of afferent C-fiber and evokes a distinct quantifiable behavior indicative of pain (paw licking/biting).30 The second phase, classified as inflammatory pain (15–30 min), is a tonic response resulting from the inflammatory processes generated by the release of inflammatory mediators, such as histamine, serotonin, prostaglandin E (PGE), and bradykinin,18 or activation of the neurons in the dorsal horns of the spinal cord.29 Centrally acting drugs (e.g., opioids) inhibit both phases, while peripherally acting drugs (e.g., nonsteroidal anti-inflammatory drugs [NSAIDs]) inhibit only the second phase. In this study, the results in Table 2 show that, in the first phase, the extract failed to inhibit the direct effect of formalin on nociceptors but morphine pretreatment inhibited paw licking in the first phase with maximum inhibition of 83.78%. However, in the second phase, A. muricata produced dose-dependent and significant (P<.001) attenuation of formalin-induced nociception with maximum inhibition of 89.76% at 200 mg/kg that was comparatively similar to the inhibition of inflammatory pain produced by morphine (96.08%) standard reference drug. The effect of the extract in this phase indicates that the extract has a possible anti-inflammatory effect. Interestingly, the antinociceptive effect elicited by A. muricata in the second phase of formalin test was reversed by pretreatment of mice with naloxone (opioid receptor antagonist)16 (Table 3), suggesting that an activation of opioid receptors and/or an increase in endogenous opioids might be involved in the antinociceptive effect of the extract. In an effort to further elucidate the mechanism of antinociceptive effect of A. muricata fruit extract, involvement of ATP-sensitive K+ channels was investigated. Previous reports showed that glibenclamide specifically blocks ATP-sensitive K+ channels, with no effect on Ca2+ or voltage-dependent K+ channels.31 In this study, the antinociceptive effect elicited by the fruit extract was not affected by pretreatment of mice with glibenclamide (KATP channel antagonist) as shown in Table 3 in formalin test. But, in agreement with the previous studies by Alves et al.,31 glibenclamide pretreatment completely reversed the antinociceptive effect of diclofenac (NSAIDs; on inflammatory pain in formalin test) (Table 3). This result rules out the involvement of ATP-sensitive K+ channels in the antinociceptive effect of A. muricata.
Table 1.
Effect of Annona muricata Fruit Extract Against Acetic-Acid-Induced Writhing Test in Mice
| Treatment | Mean number of writhes in 20 min | % Inhibition |
|---|---|---|
| Vehicle (mL/kg) | ||
| 10 | 73.20±6.06 | — |
| A. muricata (mg/kg) | ||
| 50 | 63.20±1.53 | 13.66 |
| 100 | 50.80**±2.59 | 30.6 |
| 200 | 32.80**a±0.80 | 55.19 |
| Diclofenac (mg/kg) | ||
| 20 | 29.00**±0.71 | 60.38 |
Values are expressed as mean±SEM; **P<.01 versus vehicle-treated control; aP<.05 versus A. muricata 50 mg/kg–treated group using one-way ANOVA followed by Tukey's post hoc multiple-comparison test.
ANOVA, analysis of variance.
Table 2.
Effect of A. muricata Fruit Extract Against Formalin-Induced Nociception in Mice
| Duration of paw licking (sec) | Duration of paw licking (sec) | |||
|---|---|---|---|---|
| Treatment | 0–5 min | % Inhibition | 15–30 min | % Inhibition |
| Vehicle (mL/kg) | ||||
| 10 | 84.75±0.85 | — | 166.00±32.05 | — |
| A. muricata (mg/kg) | ||||
| 50 | 112.00±1.10 | — | 97.00±15.38 | 41.57 |
| 100 | 78.80±11.80 | 7.02 | 78.25*±11.57 | 52.86 |
| 200 | 83.00±7.38 | 2.06 | 17.00***a±6.00 | 89.76 |
| Morphine (mg/kg) | ||||
| 10 | 13.75***#±8.00 | 83.78 | 6.50***#±1.50 | 96.08 |
Values are expressed as mean±SEM; *P<.05, ***P<.001 versus vehicle 10 mL/kg treated; #P<.05 versus A. muricata 200 mg/kg; aP<.05 versus A. muricata 50 mg/kg treated using one-way ANOVA followed by Tukey's post hoc multiple-comparison test.
Table 3.
Mechanisms of Analgesic Effect of A. muricata
| Duration of paw licking (sec) | Duration of paw licking (sec) | ||||
|---|---|---|---|---|---|
| Treatment | Dose | 0–5 min | % Inhibition | 15–30 min | % Inhibition |
| Vehicle | 10 mL/kg | 69.75±0.85 | 73.16±2.05 | ||
| A. muricata | 200 mg/kg | 83.00±7.38 | — | 17.00***±6.00 | 76.76 |
| Naloxone | 3+10 mL/kg | 67.4±9.66 | 3.37 | 76.25±4.88 | 0 |
| Naloxone+A. muricata | 3+200 mg/kg | 68.20±13.97 | 2.22 | 72.60γ±6.42 | 0.76 |
| Naloxone+morphine | 3+10 mg/kg | 80.00±5.08 | — | 70.30±5.36 | 3.91 |
| Glibenclamide+control | 10+10 mL/kg | 65.40±7.88 | 6.24 | 56.20±7.62 | 23.18 |
| Glibenclamide+diclofenac | 10+20 mg/kg | 66.80±13.67 | 4.22 | 78.80±6.58 | 0 |
| Glibenclamide+A. muricata | 10+200 mg/kg | 77.40±12.27 | — | 23.00**#±2.05 | 68.56 |
Values are expressed as mean±SEM; **P<.01, ***P<.001 versus vehicle-treated control; γP<.001 versus A. muricata 200 mg/kg treated; #P<.01 versus glibenclamide 10 mg/kg–treated group using one-way ANOVA followed by Tukey's post hoc multiple-comparison test.
To elucidate the effect of A. muricata on supraspinally mediated nociception, hot-plate test was carried out in mice. Pretreatment of mice with A. muricata fruit extract before exposure to thermal-induced pain increased the pain threshold of the animal. Hot plate is a more complex pain model, producing two behavioral components (i.e., paw licking and jumping) considered to be supraspinally integrated responses.32 As shown in Table 4, two-way ANOVA revealed time course and dose-dependent significant [F (4, 29)=28.41, P<.001] increase in pain threshold following oral pretreatment with A. muricata with peak effect observed at 200 mg/kg with MPE of 32.03%, which is similar to antinociceptive effect of morphine (29.22% MPE) 2 h post-treatment. Thus, it confirms the central analgesic (opioid-like) effect of A. muricata. The results of this study revealed antinociceptive effect of A. muricata fruit extract in centrally and peripherally mediated models of pain in mice.
Table 4.
Effect of Annona muricata Fruit Extract Against Hot-Plate-Induced Thermal Pain in Mice
| Reaction latency (sec) | ||||||
|---|---|---|---|---|---|---|
| Treatment | Dose | 0 min | 30 min | 60 min | 90 min | 120 min |
| Vehicle | 10 mL/kg | 1.58±0.12 | 1.43±0.07 | 1.48±0.07 | 1.61±0.11 | 1.63±0.08 |
| A. muricata | 50 mg/kg | 1.39±0.14 | 1.82±0.32 | 2.43±0.13 | 2.73*±0.13 | 1.56±0.07 |
| (5.02) | (12.10) | (15.52) | (1.97) | |||
| 100 mg/kg | 1.64±0.14 | 2.67±0.30 | 2.81**±0.27 | 2.67±0.09 | 3.46***±0.71 | |
| (12.30) | (14.04) | (12.27) | (21.72) | |||
| 200 mg/kg | 1.26±0.14 | 3.82***±0.47 | 3.03**±0.43 | 2.83*±0.22 | 4.22***a±0.31 | |
| (29.33) | (20.32) | (17.94) | (32.03) | |||
| Morphine | 10 mg/kg | 1.38±0.21 | 3.37***±0.48 | 3.78***±0.49 | 3.34***±0.12 | 3.90***±0.34 |
| (23.17) | (27.85) | (22.82) | (29.22) | |||
Values are expressed as mean±SEM; *P<.05, **P<.01, ***P<.001 versus vehicle-treated control; aP<.05 versus A. muricata 50 mg/kg, statistical level of significance by two-way ANOVA followed by Bonferroni post hoc multiple-comparison test. Values in parenthesis are % MPE.
MPE, maximum possible effect.
Due to the folkloric use of A. muricata in rheumatism and its potent inhibitory effect in second phase of formalin test, the anti-inflammatory property of A. muricata was investigated in acute models of inflammation induced by carrageenan and xylene. Carrageenan-induced paw edema in the rat hind paw is due to various mediators, such as histamine, 5-hydroxytryptamine (5-HT), bradykinin, and prostaglandins (PGs),33 which operate in parallel to produce this inflammatory response. In this study, intraplantar injection of carrageenan (100 μL, 1% w/v in normal saline) into the right hind paw resulted to an increase in hind paw circumference (edema) characterized by a rapid “early” phase (up to 2 h) response followed by a more sustained “late” phase (2±6 h) response that peaked 4 h postphlogistic injection (1.43±0.10 cm) (Table 5). However, pretreatment with A. muricata (50–200 mg/kg, p.o.) produced time course significant (P<.001) inhibition of edema induced by carrageenan with peak effect of 80.85% inhibition of edema at 200 mg/kg as compared with vehicle-treated control group (Table 5). During the first phase (0–1 h), all the doses tested significantly inhibited edema formation (P<.01). Interestingly, the antiedematogenic effect of A. muricata was effective up to 6 h postcarrageenan injection [F (4, 29)=94.83, P<.0001], which was similar to the antiedematogenic effect of diclofenac. Carrageenan-induced paw edema has been shown to be a biphasic event.34 Findings from this study show that the lyophilized fruit extract of A. muricata inhibited the initial phase of edema (0–1 h), which has been attributed to the release of histamine, 5-HT, and bradykinin.33 So also, the second accelerating phase of swelling (1–6 h), which has been well correlated with the elevated production of PG,35 and induction of inducible COX-2 in the hind paw36 was equally protected by the fruit extract. The carrageenan-induced hind paw edema in rat is known to be sensitive to COX inhibitors, but not to lipoxygenase inhibitors, and has been used to evaluate the effect of NSAIDs that primarily inhibit the COX involved in PG synthesis.37 It has been demonstrated that the suppression of carrageenan-induced inflammation after the third hour correlates reasonably with therapeutic doses of most clinically effective anti-inflammatory agents.37 In addition, in a separate series of experiments, possible inhibition of nitric oxide release in the mechanisms of the anti-inflammatory effect of the extract was also investigated (in vivo and in vitro). Pretreatment with L-NNA (20 mg/kg, i.p., nitric oxide synthase inhibitor) produced time course [F (2, 17)=58.23, P=.0004] inhibition of edema formation (Table 6), which is in agreement with the study of Salvemini et al.38 Moreover, coadministration of L-NNA and A. muricata revealed an additive anti-inflammatory effect. The enhanced anti-inflammatory action of A. muricata in the presence of L-NNA may involve inhibition of inducible NO production.38 Interestingly, in vitro assay in this study showed that A. muricata and ascorbic acid produced concentration-dependent attenuation of nitric oxide generation (in vitro) with an IC50 of 76.50 μg/mL (Y=0.8304×X+3.145) and 41.50 μg/mL (Y=0.5869×X+26.07), respectively. Moreover, there was a strong correlation between inhibitory effect of A. muricata (R2=0.98) and ascorbic acid (R2=0.97) on nitric oxide generation.
Table 5.
Effect of A. muricata Against Carrageenan-Induced Rat Paw Edema
| Change in paw circumference (cm) after treatment | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Dose | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h |
| Vehicle | 10 mL/kg | 0.88±0.10 | 1.05±0.09 | 1.23±0.06 | 1.43±0.10 | 1.33±0.05 | 1.18±0.06 |
| A. muricata | 50 mg/kg | 0.56*±0.05 | 0.85±0.07 | 1.10±0.07 | 1.30±0.11 | 1.08±0.10 | 0.78±0.06* |
| 34.29 | 19.05 | 10.20 | 8.77 | 18.87 | 34.04 | ||
| 100 mg/kg | 0.45**±0.03 | 0.65**±0.03 | 0.80*±0.04 | 0.93*±0.03 | 0.66**±0.03 | 0.43**±0.05 | |
| 48.57 | 38.10 | 34.69 | 35.09 | 49.06 | 63.83 | ||
| 200 mg/kg | 0.45**±0.03 | 0.63**±0.05 | 0.73**±0.05 | 0.68**±0.11 | 0.43**±0.12 | 0.23***±0.10 | |
| 48.57 | 40.48 | 40.82 | 52.63 | 67.92 | 80.85 | ||
| Diclofenac | 20 mg/kg | 0.43**±0.09 | 0.63**±0.09 | 0.70**±0.07 | 0.73**±0.075 | 0.50***±0.058 | 0.35***±0.07 |
| 51.43 | 40.48 | 42.86 | 49.12 | 62.26 | 70.21 | ||
Values are expressed as mean±SEM; statistical level of significance *P<.05, **P<.01, ***P<.001 versus distilled water vehicle–treated group using two-way ANOVA followed by Bonferroni post hoc multiple-comparison test. Bold values indicate percentage inhibition of edema.
Table 6.
Influence of NG-Nitro-l-Arginine on Anti-Inflammatory Effect of A. muricata Fruit Extract
| Change in paw circumference (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Dose | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h |
| Vehicle | 10 mL/kg | 0.73±0.10 | 1.05±0.03 | 1.18±0.03 | 1.23±0.08 | 1.03±0.08 | 0.83±0.11 |
| L-NNA | 20 mg/kg | 0.45±0.13 | 0.58**±0.12 | 0.68**±0.12 | 0.58***±0.09 | 0.53**±0.08 | 0.45*±0.09 |
| % Inhibition | 37.93 | 45.24 | 42.55 | 53.06 | 48.78 | 45.45 | |
| L-NNA+AM | 20+200 mg/kg | 0.40±0.08 | 0.48***±0.09 | 0.58***±0.09 | 0.60***±0.07 | 0.55**±0.09 | 0.40**±0.11 |
| % Inhibition | 44.83 | 54.76 | 51.06 | 51.02 | 46.34 | 51.52 | |
Values are expressed as mean±SEM. Statistical level of significance, *P<.05, **P<.01, ***P<.001 as compared with vehicle control–treated using two-way ANOVA followed by Bonferroni post hoc multiple-comparison test. Bold values indicate percentage inhibition of edema.
L-NNA, NG-nitro-l-arginine.
In the present study, the effect of A. muricata on acute exudative inflammation was done using the xylene-induced ear edema in mice. Xylene-induced ear edema model is useful for the evaluation of anti-inflammatory topical steroids and nonsteroidal antiphlogistic agents, especially those inhibiting phospholipase A2.39 Xylene application causes instant irritation of the mouse ear, which leads to fluid accumulation and edema, characteristic of the acute inflammatory response, suggesting the role of xylene in neurogenous inflammation.40 Suppression of this response is a likely indication of anti-inflammatory effect. The results of this study revealed that topical application of 30 μL xylene to the inner surface of the right ear increased the ear weight by 34.00±3.00 mg in vehicle-treated control group. However, the increase in ear weight was significantly (P<.001) reduced to 6.00±2.00, 8.00±2.00, and 18.00±3.00 mg, respectively, by 50 and 100 mg/kg A. muricata and 20 mg/kg prednisolone (steroidal anti-inflammatory drug) treatment (Table 7). Prednisolone was used as standard reference drug in this assay because steroidal anti-inflammatory drugs are more sensitive to the xylene-induced ear edema than NSAIDs.41 In the present study, the possible inhibitory effect of A. muricata to both COX isoforms (COX-1 and COX-2) was evaluated colorimetrically by monitoring the appearance of oxidized TMPD at 590 nm using plate reader (in vitro). Aspirin (nonselective COX inhibitor) and celecoxib (selective COX-2 inhibitor) were used as standard reference. AM, aspirin, and celecoxib were found to have potent inhibitory effect on COX-2 with an IC50 value of 69.56±3.76, 24.54±1.98, and 47.35±2.32 μg/mL, respectively, and COX-1 inhibitory concentration of (IC50 values) 88.37±4.81, 33.96±3.14, and 42.95±1.67 μg/mL, respectively. The colorimetric in vitro assay also revealed concentration-dependent increase in percentage inhibition of both COX isoforms by AM, which was comparable to the inhibitory effect of celecoxib. COX-2 inhibition was more prominent with peak effect at 200 μg/mL (Table 8). The ability of the extract to produce time course inhibition of edema induced by carrageenan and colorimetric inhibition of COX activity suggests an anti-inflammatory effect of A. muricata through inhibition of proinflammatory release.
Table 7.
Effect of A. muricata Fruit Extract Against Xylene-Induced Ear Edema
| Treatment | Change in the weight of ear (mg) | % Inhibition |
|---|---|---|
| Vehicle (mL/kg) | ||
| 10 | 34.00±3.00 | — |
| A. muricata (mg/kg) | ||
| 50 | 6.00***a±2.00 | 82.35 |
| 100 | 8.00***a±2.00 | 76.47 |
| 200 | 24.00±3.00 | 29.41 |
| Prednisolone (mg/kg) | ||
| 20 | 18.00***±3.00 | 47.06 |
Values are expressed as mean±SEM (n=6). Statistical level of significance ***P<.001 as compared with vehicle 10 mL/kg control–treated group; aP<.05 as compared with prednisolone 20 mg/kg–treated group using one-way ANOVA followed by Tukey's post hoc multiple-comparison test.
Table 8.
Percentage Inhibition of Cyclooxygenase Activities
| % Inhibition COX-1 | % Inhibition COX-2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 12.5 μg/mL | 50 μg/mL | 100 μg/mL | 200 μg/mL | 12.5 μg/mL | 50 μg/mL | 100 μg/mL | 200 μg/mL |
| Aspirin | 26.51±0.17 | 33.79±0.42 | 42.76±1.47 | 48.56±1.52 | 50.16±0.94 | 52.32±0.62 | 57.09±0.55 | 64.16±1.34 |
| Celecoxib | 20.67±0.42 | 24.71±0.94 | 30.18±2.19 | 34.15±0.96 | 26.37±0.89 | 51.54±0.27 | 53.98±1.72 | 67.86±2.18 |
| AM | 22.89±0.17 | 32.90±0.77 | 29.32±0.32 | 35.90±1.85 | 29.18±1.88 | 47.33±1.22 | 55.19±0.46 | 63.37±0.96 |
Values are expressed as percentage mean±SEM (n=3).
COX, cyclooxygenase.
Acute toxicity testing showed that A. muricata up to 4000 mg/kg did not induce any form of mortality nor any visible signs of toxic behaviors. According to OECD test guidelines on acute oral toxicity TG 420,42 no dose-related toxicity should be considered above 4 g/kg body weight. Also, our previous subchronic toxicity assay showed that the fruit extract is relatively safe for consumption.43 In view of this assertions, the fruit extract of A. muricata can be considered safe when administered via the oral route.
In conclusion, findings from the results of this study showed that A. muricata possesses analgesic and anti-inflammatory activities in various models. The analgesic effect involves interaction with peripheral mechanisms and opioid system, whereas the anti-inflammatory property involves inhibition of COX activity and nitric oxide generation. Hence justify its use in traditional African medicine in the management of painful conditions and inflammatory disorders.
Acknowledgments
The authors sincerely acknowledge Dr. A.K. Oloyo (Department of Physiology) for the kind gift of L-NNA and Mr. C.C. Micah of the Department of Pharmacology, Therapeutics and Toxicology, College of Medicine, University of Lagos, for his technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aziz PA, Yusof S: Physico-chemical characteristics of soursop fruit (Annona muricata) during growth and development. Asean Food J 1994;9:147–150 [Google Scholar]
- 2.Peters M, Badrie N, Comissiong E: Processing and quality evaluation of soursop (Annona muricata L.) nectar. J Food Qual 2001;24:361–374 [Google Scholar]
- 3.Cijo George V, Naveen Kumar DR, Rajkumar V, Suresh PK, Ashok Kumar R: Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata Linn. in normal and immortalized human cell lines. Asian Pac J Cancer Prev 2012;13:699–704 [DOI] [PubMed] [Google Scholar]
- 4.Watt JM, Breyer-Brandwijk MJ: The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed., Edinburgh and London, E&S Livingstone Ltd, 1962, pp. 58–59 [Google Scholar]
- 5.Taylor L. Technical Data Report for Graviola, Annona muricata, Vol. 10 Austin, Sage Press, 2002, pp. 1–6 [Google Scholar]
- 6.Lans CA. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed 2006;2:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan SS, Chang HL, Chen HW, et al. : Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Baxand caspase-3-related pathway. Life Sci 2003;72:2853–2861 [DOI] [PubMed] [Google Scholar]
- 8.Liaw CC, Change FR, Lin CY, et al. : New cytotoxic monotetrahydrofuran annonaceous acetogenins from Annona muricata. J Nat Prod 2002;65:470–475 [DOI] [PubMed] [Google Scholar]
- 9.Pardhasaradhi BV, Reddy M, Ali AM, et al. : Differential cytotoxic effects of Annona squamosa seed extracts on human tumour cell lines: role of reactive oxygen species and glutathione. J Biosci 2005;30:237–244 [DOI] [PubMed] [Google Scholar]
- 10.Magadula JJ, Innocent E, Otiewo JN: Mosquito larvicidal and cytotoxic activities of 3 Annona species and isolation of active principles. J Med Plants Res 2009;3:674–680 [Google Scholar]
- 11.NIH. Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academy Press, Washington, 2005, pp. 106–123 [Google Scholar]
- 12.Amida MB, Yemitan OK, Adeyemi OO: Toxicological assessment of the aqueous root extract of Sanseviera liberica Gerome and Labroy (Agavaceae). J Ethnopharmacol 2007;113:171–175 [DOI] [PubMed] [Google Scholar]
- 13.Ishola IO, Akindele JA, Adeyemi OO: Analgesic and anti-inflammatory effect of methanolic root extract of Cnestis ferruginea Vahl DC. J Ethnopharmacol 2011;135:55–62 [DOI] [PubMed] [Google Scholar]
- 14.Koster RM, Anderson M, De-Beer EJ: Acetic acid for analgesic screening. Fed Proc 1959;18:412–418 [Google Scholar]
- 15.Eddy NB, Leimbach D: Synthetic analgesics. II. Dithienylbutenyl and dithienylbutilamines. J Pharmacol Exp Ther 1959;107:385–393 [PubMed] [Google Scholar]
- 16.Rajendran NN, Thirugnanasambantham P, Viswanathan S, Parvathavarthini S, Ramaswamy S: Antinociceptive pattern of flavone and its mechanism as tested by formalin assay. Ind J Exp Biol 2000;38:182–185 [PubMed] [Google Scholar]
- 17.Venkataramanan PE, Parvathavarthini S, Viswanathan S: Role of ATP sensitive potassium channel on 7-hydroxy flavone induced antinociception and possible association with changes in glycaemic status. Ind J Exp Biol 2000;38:1172–1174 [PubMed] [Google Scholar]
- 18.Hunskaar S, Hole K: The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 1987;30:103–114 [DOI] [PubMed] [Google Scholar]
- 19.Winter CA, Risley EA, Nuss GW: Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547 [DOI] [PubMed] [Google Scholar]
- 20.Bamgbose SOA, Noamesi BK. Studies on cryptolepine II: inhibition of carrageenan-induced edema by cryptolepine. Planta Med 1981;42:392–396 [DOI] [PubMed] [Google Scholar]
- 21.Adeyemi OO, Yemitan OK, Afolabi L: Inhibition of chemically induced inflammation and pain by orally and topically administered leaf extract of Manihot esculenta Crantz in rodents. J Ethnopharmacol 2008;119:6–11 [DOI] [PubMed] [Google Scholar]
- 22.Nunez Guillen ME, Emim JA, Souccar C, Lapa AJ: Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int J Pharmacogn 1997;35:99–104 [Google Scholar]
- 23.Akindele AJ, Adeyemi OO: Anti-inflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia 2007;78:25–28 [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi Y, Shirahase H, Kunishiro K, Ichikawa A, Kanda M, Uehara Y: Synergistic effect of nitric oxide synthase and cyclooxygenase inhibitors on carrageenan-induced paw edema in rats. Arzneimittelforschung 2006;56:695–699 [DOI] [PubMed] [Google Scholar]
- 25.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L: The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun 1994;201:748–755 [DOI] [PubMed] [Google Scholar]
- 26.Bentley GA, Newton SH, Starr J: Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol 1983;79:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro RA, Vale ML, Thomazzi SM, et al. : Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 2000;387:111–118 [DOI] [PubMed] [Google Scholar]
- 28.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K: The formalin test: an evaluation of the method. Pain 1992;51:5–17 [DOI] [PubMed] [Google Scholar]
- 29.Shibata M, Ohkubo T, Takahashi H, Inoki R: Modified formalin test: characteristic biphasic pain response. Pain 1989;38:347–352 [DOI] [PubMed] [Google Scholar]
- 30.Heapy CG, Jamieson A, Russell NJW: Afferent C-fiber and A-delta activity in models of inflammation. Br J Pharmacol 1987;90:164 [Google Scholar]
- 31.Alves DP, Tatsuo MA, Leite R, Duarte ID: Diclofenac-induced peripheral antinociception is associated with ATP-sensitive K+ channels activation. Life Sci 2004;74:2577–2591 [DOI] [PubMed] [Google Scholar]
- 32.Le Bars D, Gozariu M, Cadden SW: Animal models of nociception. Pharmacol Rev 2001;53:597–652 [PubMed] [Google Scholar]
- 33.Di Rosa M, Giroud JP, Willoughby DA: Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 1971;104:15–29 [DOI] [PubMed] [Google Scholar]
- 34.Vinegar R, Schreiber W, Hugo R: Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther 1969;166:96–103 [PubMed] [Google Scholar]
- 35.Crunkhorn P, Meacock SC: Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 1971;42:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seibert K, Zhang Y, Leahy K, et al. : Pharmacological and biochemical demonstration of the role of cyclooxygenase-2 in inflammation and pain. Proc Natl Acad Sci USA 1994;91:12013–12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mequanint W, Makonnen E, Urga K: In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J Ethnopharmacol 2011;134:32–36 [DOI] [PubMed] [Google Scholar]
- 38.Salvemini D, Wang ZQ, Wyatt PS, et al. : Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 1996;118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaninir JC, Medeiros YS, Cruz AB, Yunes RRA, Calixto JB: Action of compounds from Mandevilla velutina on croton oil induced ear edema in mice; A comparative study with steroidal and non-steroidal anti-inflammatory drugs. Phytother Res 1992;6:1–5 [Google Scholar]
- 40.Richardson JD, Vasko MR: Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 2002;302:839–845 [DOI] [PubMed] [Google Scholar]
- 41.Carlson RP, O'Neill-Davis L, Chang J, Lewis AJ: Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 1985;17:197–204 [DOI] [PubMed] [Google Scholar]
- 42.OECD: The OECD (The Organisation for Economic Co-operation and Development) Guideline for Testing of Chemicals: 420 Acute Oral Toxicity OECD, Paris, 2001, pp. 1–14 [Google Scholar]
- 43.Awodele O, Ishola IO, Ikumawoyi VO, Akindele AJ, Akintonwa A: Toxicological evaluation of the lyophilized fruit juice extract of Annona muricata Linn. (Annonaceae) in rodents. J Basic Clin Physiol Pharmacol 2013;18:1–11 [DOI] [PubMed] [Google Scholar]


