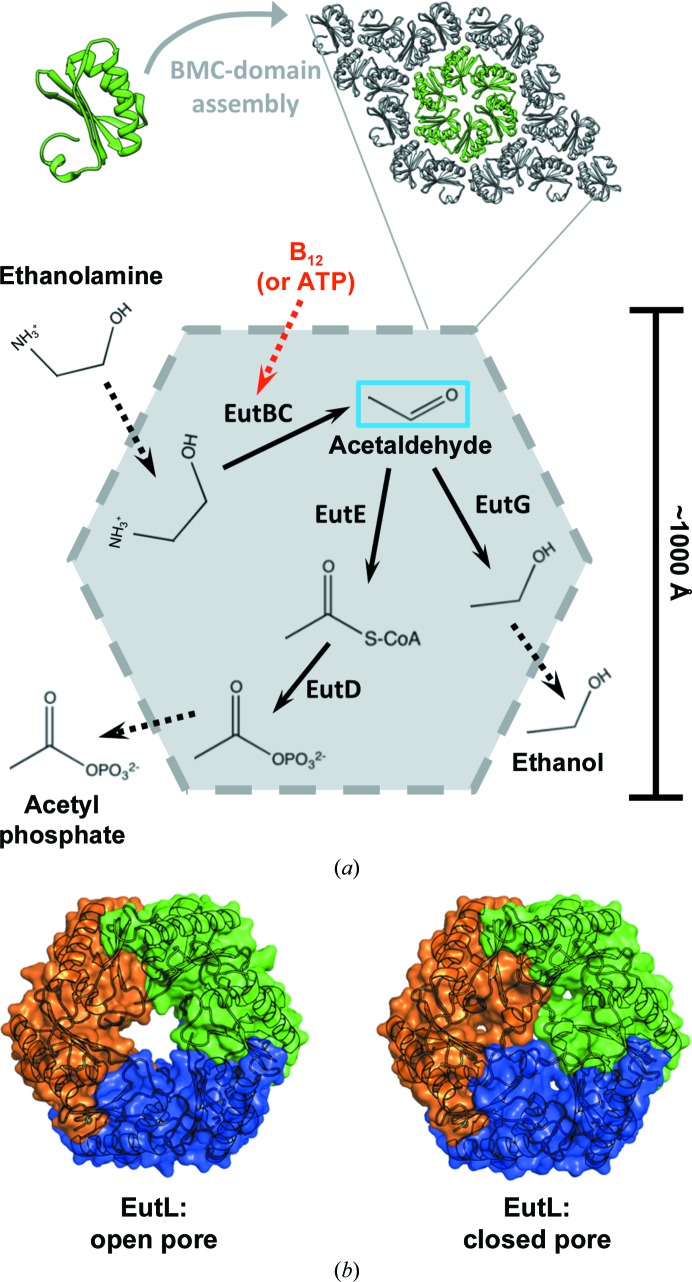
Figure 1.
The ethanolamine-utilization (Eut) microcompartment. (a) BMC-domain shell protein hexamers, such as EutL, interact along their edges, forming a thin, but tightly packed, two-dimensional layer. Flat facets combine to create a polyhedral shell assembled around a series of internal enzymes that are responsible for the initial steps of ethanolamine catabolism. The first enzymatic step of the encapsulated pathway, catalyzed by EutBC, produces a volatile intermediate (acetaldehyde; blue box) and requires a vitamin B12 (cobalamin) cofactor. The need to regenerate this cofactor implies that the shell allows the passage of molecules much larger than the substrates and products of the encapsulated enzymes (red dashed arrow). (b) EutL is a tandem BMC-domain polypeptide that forms a pseudohexameric trimer. The previous observation of both ‘open-pore’ and ‘closed-pore’ conformations of E. coli EutL indicated that this shell protein is likely to function in the gated transport of large cofactor molecules.