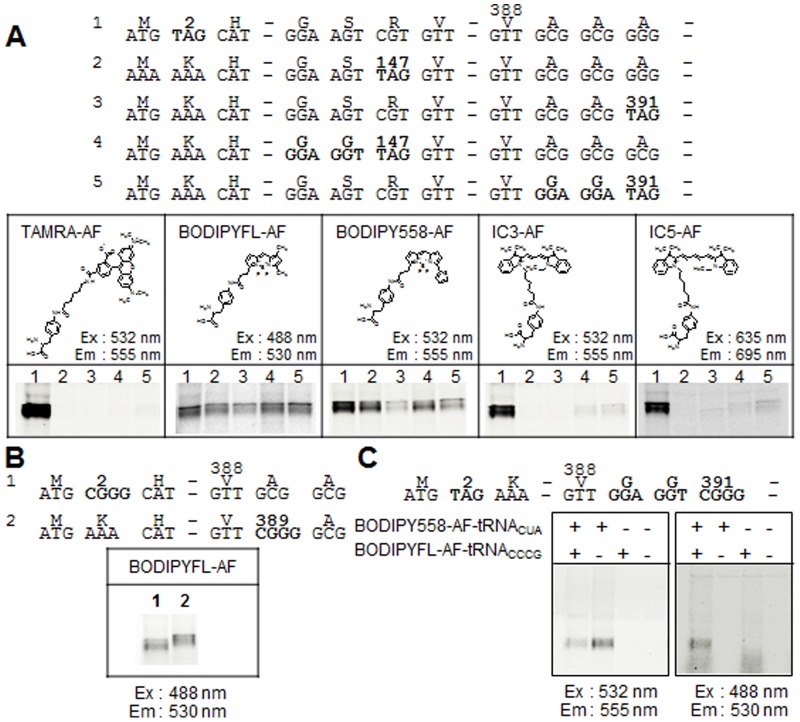
Figure 5. Incorporation of fluorescent non-natural amino acids into NhaA.
(A) Five version of NhaA (1–5) with introduced amber (TAG) codons (or Gly-Gly-TAG, introduced changes in bold) that enable incorporation of fluorescent non-natural amino acids into the N-terminal, middle or C-terminal regions of the protein (top panel) were expressed in the insect cell-free translation and translocation system in the presence of different fluorescent non-natural amino acids. The valine (V388) at the C-terminal end of NhaA was fused to additional sequences in the linker of the vectors (Table 1). Labeled NhaA protein was subjected to SDS-PAGE and fluorescent images were taken at the indicated excitation and emission wavelengths (bottom panel). The structure of the different fluorescent non-natural amino acids used in the experiments is shown above the gel images. (B) Incorporation of fluorescent non-natural amino acids into N-terminal or C-terminal positions in NhaA at an introduced four-base codon (CGGG) (top panel). NhaA was synthesized using the cell-free translation and translocation system in the presence of BODIPYFL-AF, subjected to SDS-PAGE and fluorescent images taken at the indicated excitation and emission wavelengths (bottom panel). (C) Double-labeling of NhaA by incorporation of BODIPY558-AF at an amber codon introduced into the N-terminal region and BODIPYFL-AF at the CGGG four-base codon introduced into the C-terminal region (top panel). NhaA was synthesized using the cell-free translation and translocation system in the presence of one or both fluorescent amino acids. Images of the SDS-PAGE were taken at the two different excitation and emission wavelengths indicated (bottom panel).