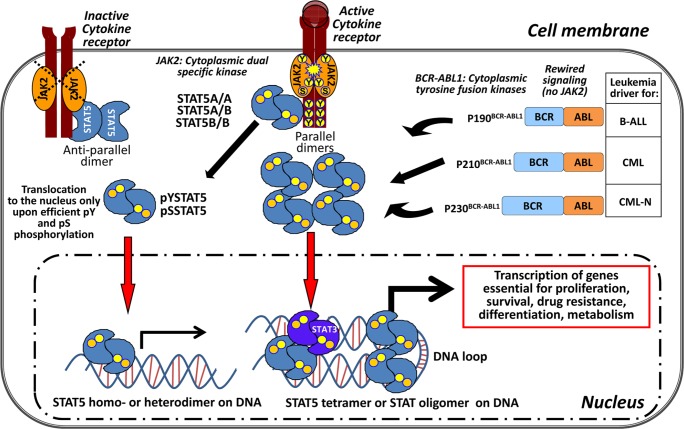
Figure 1. STAT5 – the central signaling node in BCR-ABL1+ leukemia.
Canonical activation of STAT5 starts at the cell membrane with cytokine binding to specific receptors. Receptor dimers undergo conformational changes followed by pY phosphorylation and activation of the receptor-associated tyrosine kinase JAK2. JAK2 mediates phosphorylation of the receptor at the cytoplasmic end at specific sites. STAT5 can bind to the receptors as preformed parallel and anti-parallel dimers regardless of its phosphorylation status and is activated by JAK2. The activated STAT5 proteins form parallel homo- or hetero (STAT5A/A; STAT5A/B; STAT5B/B) dimers via their SH2 domains and translocate to the nucleus to bind to the DNA. In BCR-ABL1-induced leukemias a translocation between chromosomes 9 and 22 occurs and results in a constitutive active cytoplasmic tyrosine fusion kinase. Depending on the break-point within the BCR gene three predominant variants of the BCR-ABL1 kinase are known: p190BCR-ABL1, p210BCR-ABL1, p230BCR-ABL1. The associated leukemias are shown next to the different fusion kinases. BCR-ABL1 activity rewires the JAK/STAT signaling in the leukemic cell. STAT5 is directly pY phosphorylated by BCR-ABL1 which makes JAK2 signaling dispensable. STAT5 tetramers and STAT oligomers (with activated STAT3 dimers) bind to the DNA and result in elevated target gene expression for the indicated cellular processes in a leukemic cell.