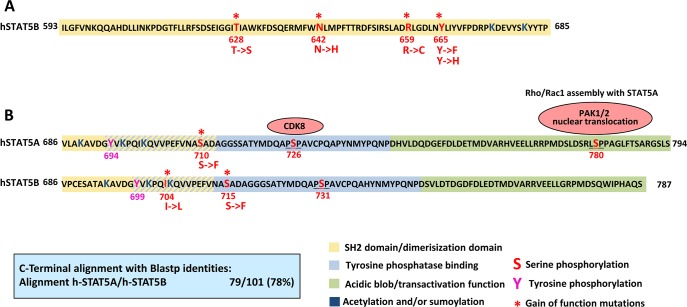
Figure 2. Gain of function mutations in STAT5.
(A) The SH2/dimerization domain (yellow) of STAT5B ranges from 593 to 712 amino acids [105]. So far, somatic mutations in the STAT5B SH2 domain have been described in LGL, T-ALL, T-PLL and HSTL. Asterisks indicate the GOF mutation position. (B) The C-terminus of STAT5A and B is the most divergent part and shares 78% sequence identity between the two closely related proteins. Lysines (K- dark blue) nearby and in the tyrosine phosphatase binding domain (light blue) undergo acetylation or sumoylation, which positively or negatively regulates pYSTAT5, respectively [106]. Apart from tyrosines 694/699 (pink), serines sites (red) 726/780 in STAT5A are constitutively phosphorylated and crucial for leukemic transformation. As upstream kinases CDK8 and PAKs have been identified. GOF mutations have been described for S710/S715 in retro virally induced screening methods and I704 in T-ALL. The transactivation domain (green) is rich in aspartic (D) and glutamic acid (E) forming a highly negatively charged region, the acidic blob, which interacts with other factors of the transcriptional machinery.