Abstract
Gene therapy is one of the frontiers of modern medicine. Adeno-associated virus (AAV)-mediated gene therapy is becoming a promising approach to treat a variety of diseases and cancers. AAV-mediated cancer gene therapies have rapidly advanced due to their superiority to other gene-carrying vectors, such as the lack of pathogenicity, the ability to transfect both dividing and non-dividing cells, low host immune response, and long-term expression. This article reviews and provides up to date knowledge on AAV-mediated cancer gene therapy.
Keywords: adeno-associated virus, cancer, cancer gene therapy, gene vehicle
1. Introduction
Cancer is still one of the most devastating diseases in the world. According to the Centers for Disease Control and Prevention (CDC), cancer incidence in the U.S.A. was 7,178,172 from 2006 to 2010, with mortality reaching 2,830,559 (www.cdc.gov/cancer/dcpc/data/). The existing therapeutic approaches, such as surgery, thermotherapy, chemotherapy, and radiotherapy, often have severe side effects, such as cytotoxicity to normal cells and strong host immune responses. Most critically, some cancers barely respond to these therapies [1, 2] and so alternative therapeutic approaches are needed. Gene therapy is one such attempt.
Gene therapy consists of three basic steps: (i) constructing a gene-carrying vector, (ii) transferring genes into target cancer cells with the vector, and (iii) expressing gene products to kill cancer cells. Constructing an effective vector for carrying therapeutic genes is essential for successful gene therapy. Gene-carrying vectors can be divided into two categories: non-viral vectors and viral vectors. Non-viral vectors, such as naked plasmids, microbubbles, nanoparticles, liposomes, and polymers, are safe, low-cost, and offer large insert size of genes; however, in vivo gene transfection and expression is inefficient and transient, despite low immunogenicity [3]. Viral vectors, such as adenoviral vectors, retroviral vectors, and lentiviral vectors, provide effective gene transduction and expression; however, they have several disadvantages, including high immunorejection, possible tumorigenicity, uncertain insertional mutagenesis, and limited constructive sizes for gene insertion. These disadvantages have prevented translation into clinical practice. Thus, it is imperative that gene-carrying vectors have (1) high transferring ability, (2) low immunorejection, and (3) long-term gene expression [4]. Adeno-associated virus (AAV) gene-carrying vectors meet these requirements.
AAVs for cancer gene therapy are superior to other gene vectors, with relatively low host immune response, weak toxicity, and long-term gene expression. AAVs have been successfully used to deliver and transfer a variety of therapeutic genes to cancer cells, including suicide genes, anti-angiogenic genes, and immune-related genes, to inhibit tumor initiation, growth, and metastasis. Herein, we review the development and recent advances of AAV-mediated cancer gene therapy, aiming to provide up-to-date information on the clinical application of AAV-based gene therapy.
2. Biology of AAVs
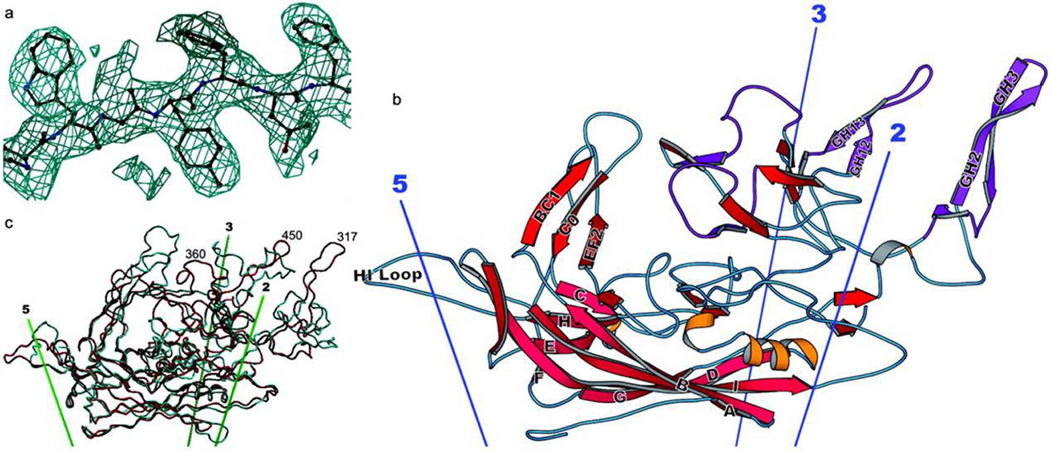
The adeno-associated virus, first discovered in the 1960s [5], is replication-deficient and belongs to the family of Parvoviridae. As the best known representative of all the AAVs, AAV2 contains a single stranded DNA genome comprising of inverted terminal repeats (ITRs) and two open reading frames encoding replication and capsid proteins. The structure of AAV2 has been determined to 3-Å resolution (Fig. 1) [6]. Recently Gao et.al have obtained more than 120 novel primate AAVs[7]. The diverse tissue and cell tropisms of mainly used AAV vectors were listed in table 1.
Fig. 1. Structure of the AAV-2 subunit and comparison with related structures.
(a) Experimental electron density for AAV-2. Phases for this 3-Å resolution electron density map are independent of the AAV-2 model, having been obtained by symmetry averaging and extension from a CPV model at 15-Å resolution. Density is clear and allows an unambiguous fitting of the chemical sequence throughout VP3. (b) Ribbon drawing of the AAV-2 subunit. The locations of the neighboring symmetry axes are shown. The β-barrel is on the inner surface of the capsid (pink) with strands of the two sheets labeled conventionally as A, B, I, D, and G, and C, H, E, and F. Loops are labeled according to the flanking strands—e.g., GH loop. Regions where the sequence differs greatest between the AAV serotypes are colored purple [122]. (c) Comparison of the backbones of AAV-2 (red) and canine parvovirus (cyan). The loop structure, which is responsible for many of the viral-host interactions differs substantially between AAV-2 and canine parvovirus, is largely absent from insect densoviruses (not shown). Reprinted with permission from ref 6.
Table 1.
The different tissue and cell tropisms of mainly used AAV serotypes.
| Serotypes | Origins | Tropisms | References |
|---|---|---|---|
| AAV1 | Simian sources | Skeletal muscle, spinal cord, heart | [8], [9] |
| AAV2 | Human clinical specimens | CNS, retina, ubiquitous | [10], [11], [12] |
| AAV3 | Human clinical specimens | Cochlear inner hair cells, liver cancer cells | [13], [14] |
| AAV4 | Simian sources | Heart, lung | [15] |
| AAV5 | Human clinical specimens | Apical airway cells, liver cells | [16] |
| AAV6 | Recombinant of AAV2(5’) and AAV1(3’) | Apical airway cells | [17] |
| AAV7 | Rhesus monkey | Retina, muscle, liver | [18] |
| AAV8 | Rhesus monkey | Skeletal and cardiac muscles, retina | [19], [20] |
| AAVrh.8 | Rhesus monkey | Muscle, liver | [7] |
| AAV9 | Human sources | CNS, peripheral tissues, | [7, 21] |
| AAV10 | Cynomolgus monkeys | Mouse heart, lung, monkeys liver, kidney, and uterus | [22] |
| AAVrh.10 | Rhesus macaque | CNS, | [23] |
| AAV11 | Cynomolgus monkeys | Mouse muscles, kidney, spleen, lung, heart, and stomach | [22] |
| AAV12 | Simian sources | Muscle and salivary glands | [24] |
| AAVrh.43 | Rhesus monkey | CNS | [25] |
3. Advances of AAV vectors
The AAV based gene delivery systems are more attractive comparing to other vectors. More benefits were discovered using AAV vectors such as more safety due to the lack of pathogenicity, more varied host and cell-type tropisms, long-term gene expression, ability to transfect both dividing and nondividing cells, absence of enormous immune response. Furthermore, the discovery of more novel AAV serotypes will further extend the scope of application of AAV based gene delivery system.
However several problems about this gene delivery system should be addressed. Firstly, the effective packaging capacity of AAV is limited to 4.1 to 4.9kb [26], which restricts the transduction of larger genes. Secondly, antibody neutralization rises because of prior exposure of human beings with multiple AAV serotypes [27]. Thirdly, challenges with high-efficient transduction to specific cell populations remain in AAV mediated gene delivery system. Since these problems influenced the extended application of AAV based gene therapy, a variety of attempts to improve this vector have been carried out. Self-complementary AAV (scAAV) vectors can fold into double-stranded DNA (dsDNA) without DNA synthesis or base-pairing between multiple vector genomes [28] bypassing the conversion to dsDNA. Naturally, these vectors are more sufficient to transgene expression than normal AAV vectors. The clinical application of AAV vector was slowed down due to the limitation of packaging capacity of rAAV. Cotransduction of dual AAV vectors seems to be an alternative to solve this problem. Transgene expression cassettes are split into two, and each is packaged into a AAV vector. Then expression of full-length transgene is obtained via homologous recombination or viral inverted terminal repeat mediated recombination. Since low levels of pre-existing neutralizing antibodies significantly reduced the efficacy of therapeutic AAV gene delivery, modification of AAV capsid involved in interactions with host immunity has been an good idea to escape neutralization. Directed selection of AAV variants, “shielding” polymers, site-directed mutagenesis and directed evolution of AAV capsid made the neutralization escape of AAV based delivery system feasible [29].
4. Development of AAV-mediated gene therapy
A variety of preclinical experiments involving AAV-based gene therapies have been carried out. In 1984, a study reported the first construction of AAVs as vectors for gene transfer to eukaryotic cells [30], while the urgency of curing cystic fibrosis (CF) motivated the development of in vivo AAV-based gene therapy technology. By utilizing AAV vectors, scientists successfully transduced a therapeutic gene, cystic fibrosis transmembrane regulator (CFTR), into the airways of rabbits and monkeys. Expression of CFTR was detected for as long as 6 years in the airways. Subsequently, the first trial of AAV-mediated CF gene therapy was reported in 1995 [31]. Since then, extensive research has been done on tissue tropism, targeting cell receptors associated with infection, gene/vector delivery routes, pathophysiologic effects, and AAV vector immune profiling. The first licensed gene therapy product with an AAV vector was Glybra, specifically designed and used to supplement deficiency in lipoprotein lipase (LPL). In recent years, gene therapies with different subtypes of AAV vectors have been reported for treatments of a variety of diseases as listed in Table 2.
Table 2.
Summary of AAV-mediated gene therapies in clinical application in recent years.
| AAV serotypes |
Disease | Gene transferred | Reference |
|---|---|---|---|
| AAV1 | Pompe disease, pulmonary emphysema, LGMD2D, LPLD, cystic fibrosis, Alzheimer’s disease | hGAA, M-AAT, alpha-sarcoglycan, LPL(S447X), IL10, anti-Abeta single-chain antibody | [32], [33, 34], [35], [36], [37], [38] |
| AAV2 | Choroideremia, LCA, PD | CHM, RPE65, AADC | [39], [40], [41] |
| AAV3 | Liver cancer | Pyruvate dehydrogenase E1 alpha subunit | [14] |
| AAV4 | Huntington’s disease, ALS | BDNF and noggin, IGF-1 or VEGF-165 | [42], [43] |
| AAV5 | Cone-rod dystrophy, polyglutamine disease | RPGRIP1, Hsp40 | [44], [45] |
| AAV6 | Heart failure, cardiac transplantation | S100A1, HO-1 | [46], [47] |
| AAV7 | GSD-1a | G6Pase | [48] |
| AAV8 | Hemophilia B, muscular dystrophies, | hFIXco, VEGF-C/D | [49], [50] |
| AAV9 | Alzheimer’s disease, heart failure, salivary hypofunction | IgVL5D3/TLK1B/, SERCA/MiR378, tousled-like kinase 1B | [51], [52], [53], [54] |
| AAVrh.10 | Friedreich’s ataxia, JNCL | Frataxin, CLN3 | [55], [56] |
Abbreviations: GAA = lysosomal acid alpha-glucosidase, AAT = α-1 antitrypsin, LGMD2D = limb-girdle muscular dystrophy type 2D, CHM = choroideremia, LCA = Leber congenital amaurosis, PD = Parkinson’s disease, AADC = aromatic amino acid decarboxylase, RPE65 = retinal pigment epithelium-specific protein 65 kDa, hFIXco = codon-optimized human factor IX, LPLD = lipoprotein lipase deficiency, TLK1B = tousled-like kinase 1B, SERCA = sarcoplasmic reticulum Ca(2+)-ATPase, BDNF = brain-derived neurotrophic factor, GSD-1a = glycogen storage disease type 1a, JNCL = juvenile neuronal ceroid lipofuscinosis, PNS = peripheral nervous system.
5. Advances in AAV-mediated cancer gene therapy
5.1. AAV-mediated suicide gene therapy
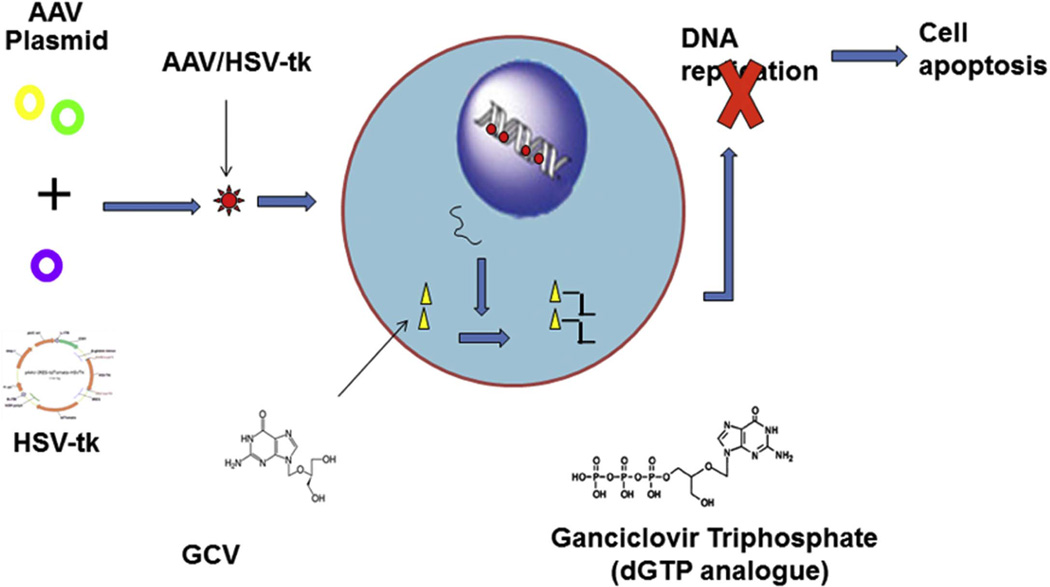
Suicide gene therapy, also called gene-directed enzyme prodrug therapy (GDEPT) or molecular chemotherapy, is currently the most promising strategy for genetic treatment of different cancers. GDEPT relies on the intratumor delivery of a transgene encoding an enzyme, which then activates a systemically-delivered prodrug that inhibits DNA polymerase and blocks DNA replication in tumor cells. Among the candidate genes, the herpes simplex virus thymidine kinase gene/ganciclovir prodrug (HSV-tk/GCV) system is an excellent example of the clinical application of GDEPT (Fig. 2) [57]. Studies have shown that the AAV-mediated HSV-tk/GCV therapeutic system generates strong antitumor efficacy [58, 59].
Fig. 2. The AAV/HSV-tk and GCV suicide gene system.
The AAV vector carrying an HSV-tk gene is constructed using RC and helper plasmids, and AAV/HSV is then transduced into the target cells. Cell killing is subsequently achieved by inhibiting the cell cycle and promoting cell apoptosis as systemically-administered GCV is converted to a GCV triphosphate.
One of the most attractive elements of the HSV-tk/GCV therapeutic system is its bystander effect, i.e., the killing of untransduced tumor cells surrounding transduced tumor cells [60]. This phenomenon is mainly caused by the free passive diffusion of antimetabolites or transfer of phosphorylated GCV molecules between cells via gap junction intercellular communication (GJIC) [61]. Connexin is an important GJIC molecule generally expressed in the cytosol. Some study groups have demonstrated that overexpression of connexin43 restored GJIC in tumor cells and thereby resulted in the increased antitumor effect of the HSV-tk/GCV system [62, 63]. Recently, a novel family of GJIC named Pannexin (Panx) [64]may play the same roles as the connexins[65].
5.2. AAV-mediated antiangiogenesis gene therapy
Angiogenesis often accompanies the growth of tumors to provide oxygen and nutrients, causing rapid proliferation of cancer cells. Antiangiogenic therapy was developed to starve tumor cells [66]. Vascular endothelial growth factor (VEGF), an important mediator of angiogenesis in both healthy and diseased tissues, is a crucial antitumor target. A study demonstrated that a single intravenous administration of AAV/VEGF-Trap led to long-term efficacy and permitted not only suppression of primary tumor growth but also prevention of pulmonary metastasis [67]. AAV-mediated transduction of other antiangiogenic genes, such as pigment epithelium-derived factor (PEDF) [68] [69], endostatin 34, Kringle 5 [70], and kallistatin [71], also showed significant inhibition of tumor angiogenesis, tumor growth, and metastasis. Overexpression of the specific antagonist of hepatocyte growth factor (HGF) NK4, consisting of an N-terminal hairpin domain and four kringle domains, can antagonize the HGF/c-Met system competitively, inhibit c-Met signaling and tumor metastasis, and therefore offer additional antiangiogenic activity [72].
5.3. AAV-mediated immune gene therapy
Immune gene therapy has advanced rapidly over the last decades and is becoming an important approach in the treatment of different cancers. AAV-mediated immune gene therapy is based on the successful activation of the host immune system upon transfer of therapeutic genes to targets cells, including cytokines, immunogenic cell surface molecules, and tumor antigens, as listed in Table 3.
Table 3.
Overview of AAV-based immune gene therapy in recent years.
| AAV serotypes |
Immune genes | Targeted tumors | References |
|---|---|---|---|
| AAV2 | PSA | Prostate cancer | [73] |
| AAV2 | IL-12 | Glioblastoma | [74] |
| AAV2 | IL-15 | Cervical cancer and breast cancer | [75, 76] |
| AAV8 | CCR4 | Lymphoma | [77] |
| rAAV2 | IFN-beta | Retinoblastoma | [78, 79] |
| AAV5 /AAV9 | HPV16 L1/E7 | HPV tumors | [80] |
| AAV2/6 | LMP1 and LMP2 | Nasopharyngeal carcinoma | [81] |
| AAV2 | SLC | Hepatoma | [82] |
Abbreviations: PSA = prostate specific antigen, IL-12 = interleukin-12, IL-15 = interleukin-15, CCR4 = cutaneous T lymphoid chemokine receptor 4, IFN-beta = Interferon-β, LMP = latent membrane proteins, SLC = lymphoid tissue chemokine.
5.4. Other AAV-mediated gene therapies
The negative signaling associated with programmed death-1 (PD-1) contributes to tumor evasion. Investigators delivered the extracellular domain of murine PD-1 to a tumor site using AAV vectors and obtained high antitumor efficacy [83]. Since the Fas/Fas ligand (FasL) system involves apoptosis of tumor cells, AAV-mediated transduction of human FasL genes in human laryngeal carcinoma Hep2-bearing nude mice causes obvious suppression of tumor growth and prolonged survival rates (60% vs 0% in control untreated mice) [84]. Administration of the AAV-based central cell-binding domain and C-terminal heparin-binding domain (CBD-HepII) recombinant polypeptide of human fibronectin suppresses the growth and spontaneous metastasis of breast carcinoma, and prolongs the survival of tumor-bearing mice [85]. Prostate apoptosis response-4 (Par-4) is the tumor-suppressor protein that results in the cell apoptosis in tumor cells not in normal cells. A study showed that expression of AAV/Par-4 induced rapid cell death in the HepG2 hepatocarcinoma model [86].
AAV3-mediated expression of the pyruvate dehydrogenase E1 alpha subunit gene can also result in the apoptosis of liver cancer cells [14]. Tumor necrosis factor (TNF) related apoptosis-inducing ligand (TRAIL) receptor 2 (also called death receptor 5, DR5) is usually expressed in tumor cell lines but not in normal cells. Investigators have demonstrated that an AAV-based anti-DR5 mouse-human chimeric antibody can markedly inhibit tumor growth in vitro and in vivo [87]. Survivin is an apoptosis protein. The binding of heat shock protein 90 (Hsp90) to survivin can inhibit the degradation of survivin by the ubiquitin-proteasome system. Since survivin Lys-79~Leu-87 is the binding site for Hsp90, the identical 9-amino acid peptide, named shepherdin, was constructed. AAV-mediated NT4-Ant-shepherdin, consisting of a cell-penetrating peptide (Ant), the signal peptide of neurotrophin-4 (NT4) and shepherdin, can significantly suppress the growth of the lung cancer cell line A549 by inducing apoptosis [88]. Telomerase is made of the catalytic subunit of human telomerase reverse transcriptase (hTERT) and telomerase RNA (hTR). Overexpression of a 27 kDa C-terminal polypeptide of hTERT (hTERTC27) carried by recombinant AAV can prevent the growth of human U87-MG glioblastoma cells in nude mice by increasing necrosis, apoptosis and neutrophil infiltration, and by decreasing microvascular density [89].
5.5. AAV-mediated RNA interference therapy
RNA interference (RNAi) is a therapeutic biological tool in different cancers. Several types of non-encoding RNAs (siRNAs, shRNAs, and artificial microRNAs) have been used in preclinical studies through the endogenous RNAi pathway. AAV-mediated short hairpin RNA (shRNA) is ubiquitously used in gene knockdown applications. Androgen receptor (AR) is associated with prostate cancer progression. Transduction of AAV/shRNA against AR inhibits the growth of tumors, even abrogating xenograft tumors within 10 days. Furthermore, a study demonstrated that efficacy was achieved by reducing the expression of AR-related survival genes, leading to a dramatic apoptotic response [90].
MicroRNA (miRNA) is a type of highly conserved small RNA molecule that plays an important role in the regulation of gene expression. Systemic administration of AAV-mediated miRNA-26a led to the inhibition of cancer growth, induction of tumor apoptosis, and protection from disease progression [91]. Participation of miRNA in the pathophysiology of pancreatic cancers was also reported [92]. Knockdown of Four and a half (LIM) protein 2 (FHL2) with AAV-mediated FHL2 shRNA transduction in colon cancer resulted in G0/G1 cell cycle arrest and inhibition of tumor cell growth. Delivery of scAAV2-based siRNA against the unfolded protein response inositol-requiring protein 1α (IRE1α), X-box-binding protein 1(XBP)-1, and activating transcription factor 6 (ATF6) remarkably suppresses breast tumor angiogenesis [93]. Snail is an important transcriptional factor involved in the antiapoptotic and chemoresistant phenotype of pancreatic cancer. Administration of AAV-mediated snail siRNA inhibits the growth of xenografted pancreatic cancers [94].
5.6. AAV-mediated targeting gene therapy
To date, the primary weakness of systemic delivery of AAV-based therapeutic genes is low tumor-targeting efficiency. Specificity may be improved by adding tumor-specific promoters to AAV vectors. To date, there are a variety of AAV-mediated therapeutic genes driven by various tumor-specific promoters, such as human telomerase reverse transcriptase (hTERT) [95–97], extracellular domain of tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL) [98], tumor-targeting motif, including RGD, and tenascin C (TnC) [99]. This target-specific AAV-gene therapy can significantly suppress tumor growth.
5.7. Combination treatment with multiple genes and chemotherapies
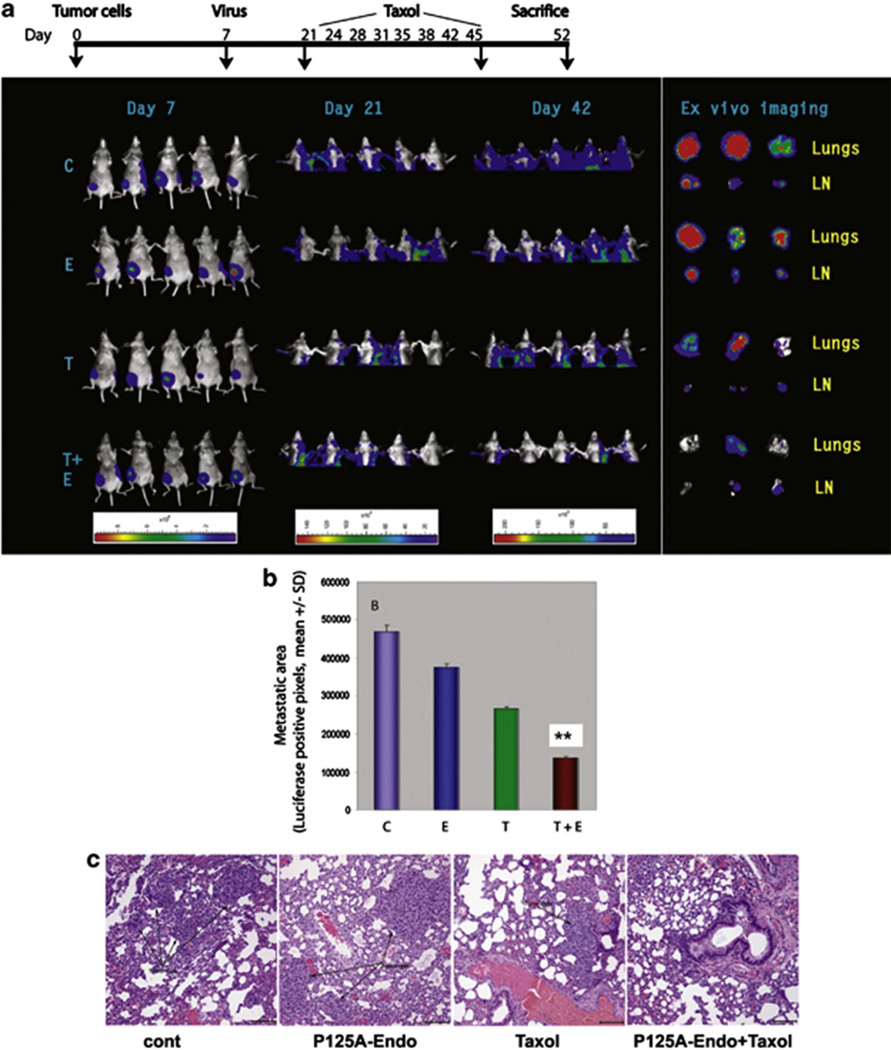
To improve antitumor efficacy, recent efforts have focused on combining AAV-mediated multi-gene therapies with chemotherapies. Several studies reported such combination treatment: AAV-based apoptin and IL24 [100], HSV-tk and endostatin [101, 102], TRAIL and cisplatin [103, 104], P125A-endostatin, a mutant endostatin, and paclitaxel (Fig. 3) [105], P125-endostain and carboplatin [106], type I interferon beta (IFN-beta) and trichostatin A (TSA) [107], the survivin mutant Thr34Ala and oxaliplatin [108]. In addition, several studies demonstrated that combining AAV-based gene therapy with radiation therapy [109] and focused ultrasound therapy [110] can significantly increase cytotoxicity to tumor cells.
Fig. 3. Effect of antiangiogenic gene therapy and paclitaxel on metastasis of orthotopically transplanted human breast cancer cells (intervention model).
MDA-MB-231-luc cells were transplanted into mammary fat pads of female, athymic mice. Whole body images were obtained on a weekly basis after intraperitoneally administration of luciferin in a Xenogen system (a). Luminescence signals from the primary tumors were too strong and were overlapping with the signals originating from the metastatic sites. Therefore, the primary tumors were shielded (lower half) to reveal tumor metastasis to lungs and lymph nodes (days 21 and 42). Intensity of signals is shown at the bottom. On day 42, mice were killed and brachial lymph nodes and lung tissues were removed for ex vivo imaging. C, adeno-associated virus (AAV)-LacZ; E, AAV-P125A-endostatin; T, paclitaxel; T+E, combination treatment; LN, lymph node. Panel (b) shows relative luciferase-positive pixels from the metastatic sites. Each value is a mean of five animals ± s.d. Panel (c) shows the histopathology of lungs. The lung tissues were stained with hematoxylin and eosin stain and the black arrow shows the tumor that has metastasized to the lungs (×200). **Denotes statistical significance (P<0.02). Reprinted with permission from ref 105.
6. Clinical trials of AAV-mediated gene therapy
Clinical applications of AAV-mediated gene therapies show great promise in the treatment of some life-threatening diseases, including hemophilia B, Leber congenital amaurosis, Parkinson's disease, and different cancers. Hemophilia B is an X-linked disorder. A clinical trial of AAV-mediated human factor IX (FIX) gene therapy was carried out in a first group of three patients in 2000, and FIX expression was detected in the muscles and circulating systems of the three subjects [111]. Subsequently, additional AAV-based FIX gene therapy trials in humans with severe hemophilia B were performed. Manno et al. carried out a phase I and II dose escalation clinical study in patients with severe hemophilia B that reached therapeutic levels of FIX with no evidence of acute or long-lasting toxicity [112]. The Nathwani group infused a single dose of AAV-based LP1-hFIX in six patients with severe hemophilia B (FIX activity, <1% of normal values). Expression of FIX at 2~11% was detected, and 80% of the patients remained free of spontaneous hemorrhage, an important manifestation of hemophilia B [49]. Meanwhile, no evidence of local or systemic toxicity of AAV-based FIX gene therapy was detected up to 40 months after gene injection [113]. Recently, AAV8 carrying a codon-optimized human FIX transgene was delivered systemically to six patients with severe hemophilia B, demonstrating long-term transgene expression of FIX with improvement of the bleeding phenotype [49]. A 10 year-long AAV-mediated human FIX expression was reported in muscles, which represents the longest transgene expression so far [114].
Alpha-1 antitrypsin (AAT) deficiency, one of the most common single-gene disorders, is suitable as a target for gene therapy. A phase I clinical trial of AAV-based M-AAT gene delivery has been validated at different doses. The M-specific AAT was expressed for 1 year, with no obvious side effects [34, 115]. Further, a study of AAV-mediated M-AAT gene transfer demonstrated that intramuscular delivery of rAAV1 –AAT led to ongoing transgene expression for at least 1 year. A regulatory T cell (Treg) response was induced, which permitted transgene overexpression [33].
Parkinson’s disease is a severe neurodegenerative disorder, clinically characterized by degeneration of dopamine neurons in substantia nigra pars compacta. Neuturin is a functional analogue of glial cell-derived neurotrophic factor (GDNF), which protects dopamine neurons from degeneration. The delivery of AAV-based neuturin promoted long-term motor function improvement in a phase I clinical trial [116]. A double-blind, randomized, controlled trial lasting for about 2 years proved that AAV2/neuturin gene therapy for moderately advanced Parkinson’s disease is safe and feasible [117]. The loss of L-dopa is one of the main causes of progressive Parkinson’s disease, a response to declining levels of aromatic amino acid decarboxylase (AADC). A clinical phase I study of AAV2-AADC gene therapy showed safe transgene expression over 4 years [118]. These AAV-based clinical trials have validated the promising potential of gene therapies in Parkinson’s disease.
Leber congenital amaurosis (LCA) is a kind of autosomal recessive blinding retinal disease that is incorrigible. A mutant of retinal pigment epithelium-specific 65-kDa (RPE65) is a major cause of LCA. A phase I clinical trial of AAV2-mediated RPE65 gene therapy improved visual function, and the 3-year follow-up clinical data confirmed that AAV-mediated RPE65 gene therapy for LCA was safe and tolerable [119]. Choroideremia (CHM) is an X-linked recessive disease caused by a mutation in the CHM gene encoding Rab escort protein 1 (REP1). AAV-based REP1 gene therapy also corrected visual acuity significantly (Fig. 4) [39].
Fig. 4. Detailed analysis of retinal function and structure in patients with choroideremia.
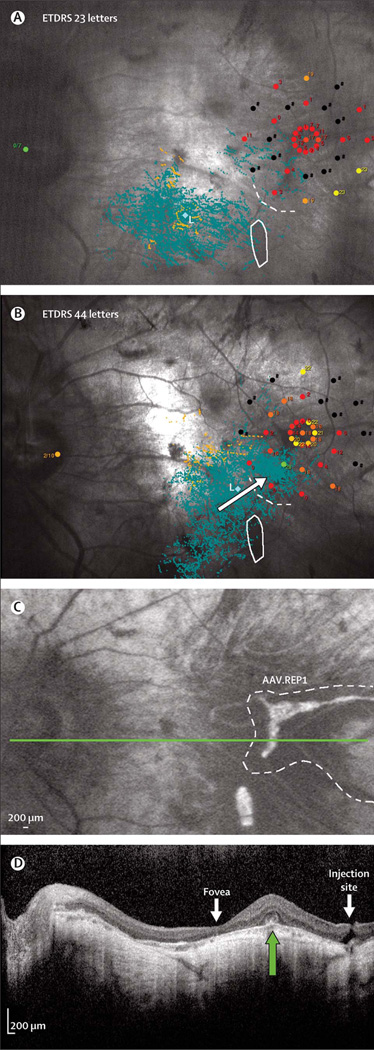
(A) Pre-surgery and (B) 6 months post-surgery analysis of patient 1 showing a shift in the cloud of variable fixation (blue dots; B, white arrow) into the area of retina exposed to vector (dotted white line), but away from the area of surviving autofluorescent retinal pigment epithelium (white solid line) that was not exposed to vector. The increase in retinal sensitivity also correlated anatomically with the region of surviving retina exposed to AAV.REP1 (C, dotted line) and residual outer retina identified with optical coherence tomography scanning (D, green arrow). The thin fovea and injection site can be seen on either side of the residual retina (green arrow). Green dot (L) is the mean center of all fixation points. ETDRS=Early Treatment for Diabetic Retinopathy Study (a standard vision test). AAV=adeno-associated virus. REP1=Rab escort protein 1. Reprinted with permissions from ref 39.
In addition to the conventional transgene therapies described above, several clinical trials of AAV-based immune therapy in cancer have also been performed. Di L et al. carried out a clinical safety trial of cytotoxic T lymphocyte (CTL) infusion, which was induced by delivery of dendritic cells (DCs) transduced with rAAV/cancer embryonic antigen (CEA) cDNA to patients with advanced cancers. This trial reported no severe side effects in almost all of the 26 patients [120]. Another phase I clinical trial evaluating the safety and efficacy of AAV-DC-CTL-CEA treatment in patients with stage IV gastric cancer has been carried out at the Tianjin Medical University Cancer Institute and Hospital (Clinical trial.gov NCT01637805). Meanwhile, a single intra-articular infusion of AAV2 carrying a human tumor necrosis factor-immunoglobulin Fc fusion gene (AAV2-TNFR:Fc) in 15 patients showed safety and tolerability [121].
7. Concluding remarks
Compared to other vectors, AAV offers several advantages, including low pathogenicity, limited immunogenicity, efficient gene transfer, and long-lasting gene expression. Tremendous efforts have led to the great development of AAV-mediated gene therapy in different cancers. Although obstacles remain, promising preclinical and clinical results have opened new avenues for the efficient management of malignancy using AAV-integrated gene therapy technology.
Highlights.
We reviews up to date knowledge on AAV-mediated gene therapy for cancer, including suicide gene therapy, antiangiogenesis gene therapy, immune gene therapy, RNA interference therapy, targeting gene therapy, and combination treatment with multiple genes and chemotherapies for cancers.
We reviews up to date knowledge on the clinical trials of AAV-mediated gene therapy.
Acknowledgments
This study was supported by National Basic Research Program of China (973 Program, 2014CB744505), US National Institutes of Health grant (R01EB012467), Natural Science Foundation of China (81401504, 81430040), Medical science and technology project of Zhejiang Province (201461368), Program for National Science and Technology Major Project of China (2013ZX10002004-001-005), Qianjiang Talent Program of Zhejiang Province in China (2012R10027), Scientific Research Foundation of the Health Bureau of Zhejiang Province in China (WKJ2012-2-030), Fundamental Research Funds for the Central Universities (2012QNA7037) and SRF for ROCS, SEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that there are no conflicts of interest.
References
- 1.Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, Racchumi J, Xu G, Wu H, Ma J, Steggerda SM, Coleman M, Leslie C, Wang YL. Ibrutinib resistance in chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:2352–2354. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas RK. Overcoming drug resistance in ALK-rearranged lung cancer. The New England journal of medicine. 2014;370:1250–1251. doi: 10.1056/NEJMe1316173. [DOI] [PubMed] [Google Scholar]
- 3.Su CH, Wu YJ, Wang HH, Yeh HI. Nonviral gene therapy targeting cardiovascular system. American journal of physiology. Heart and circulatory physiology. 2012;303:H629–H638. doi: 10.1152/ajpheart.00126.2012. [DOI] [PubMed] [Google Scholar]
- 4.Petrs-Silva H, Linden R. Advances in recombinant adeno-associated viral vectors for gene delivery. Current gene therapy. 2013;13:335–345. doi: 10.2174/15665232113136660028. [DOI] [PubMed] [Google Scholar]
- 5.Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 6.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. Journal of virology. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck B, Xiao W. Characterization of tissue tropism determinants of adeno-associated virus type 1. Journal of virology. 2003;77:2768–2774. doi: 10.1128/JVI.77.4.2768-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai LK, Chen CL, Ting CH, Lin-Chao S, Hwu WL, Dodge JC, Passini MA, Cheng SH. Systemic administration of a recombinant AAV1 vector encoding IGF-1 improves disease manifestations in SMA mice. Molecular therapy. 2014;22:1450–1459. doi: 10.1038/mt.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joniec-Maciejak I, Ciesielska A, Wawer A, Sznejder-Pacholek A, Schwenkgrub J, Cudna A, Hadaczek P, Bankiewicz KS, Czlonkowska A, Czlonkowski A. The influence of AAV2-mediated gene transfer of human IL-10 on neurodegeneration and immune response in a murine model of Parkinson's disease. Pharmacological reports. 2014;66:660–669. doi: 10.1016/j.pharep.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Ahn M, Bajsarowicz K, Oehler A, Lemus A, Bankiewicz K, DeArmond SJ. Convection-enhanced delivery of AAV2-PrPshRNA in prion-infected mice. PloS one. 2014;9:e98496. doi: 10.1371/journal.pone.0098496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Zhang L, Gu L, Lu L, Gao G, Li W, Xu G, Wang J, Gao F, Xu JY, Yao J, Wang F, Zhang J, Xu GT. Subretinal delivery of AAV2-mediated human erythropoietin gene is protective and safe in experimental diabetic retinopathy. Investigative ophthalmology & visual science. 2014;55:1519–1530. doi: 10.1167/iovs.13-13155. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, Kanazawa T, Takeuchi K, Ajalli R, Mizukami H, Kume A, Ichimura K, Ozawa K. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Molecular therapy. 2005;12:725–733. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, Liu C, Srivastava A, Stacpoole PW. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Molecular genetics and metabolism. 2009;98:289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen S, Troupes AN, Pulicherla N, Asokan A. Multiple roles for sialylated glycans in determining the cardiopulmonary tropism of adeno-associated virus 4. Journal of virology. 2013;87:13206–13213. doi: 10.1128/JVI.02109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL, Chiorini JA. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. Journal of virology. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. Journal of virology. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. The journal of gene medicine. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Molecular therapy. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Miyake N, Fujimoto C, Yaguchi C, Iijima O, Shimada T, Takahashi H, Miyake K. Adeno-associated virus type 8 vector-mediated expression of siRNA targeting vascular endothelial growth factor efficiently inhibits neovascularization in a murine choroidal neovascularization model. Molecular vision. 2014;20:488–496. [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzo A, Marco S, Garcia M, Villacampa P, Ribera A, Ayuso E, Maggioni L, Mingozzi F, Haurigot V, Bosch F. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Human gene therapy. 2012;23:1237–1246. doi: 10.1089/hum.2012.029. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Molecular therapy. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. Journal of virology. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Molecular therapy. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Human gene therapy. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 27.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene therapy. 2003;10:964–976. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- 28.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene therapy. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 29.Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharmaceutical research. 2008;25:489–499. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tratschin JD, West MH, Sandbank T, Carter BJ. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Molecular and cellular biology. 1984;4:2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Human gene therapy. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 32.Smith BK, Collins SW, Conlon TJ, Mah CS, Lawson LA, Martin AD, Fuller DD, Cleaver BD, Clement N, Phillips D, Islam S, Dobjia N, Byrne BJ. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Human gene therapy. 2013;24:630–640. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller C, Chulay JD, Trapnell BC, Humphries M, Carey B, Sandhaus RA, McElvaney NG, Messina L, Tang Q, Rouhani FN, Campbell-Thompson M, Fu AD, Yachnis A, Knop DR, Ye GJ, Brantly M, Calcedo R, Somanathan S, Richman LP, Vonderheide RH, Hulme MA, Brusko TM, Wilson JM, Flotte TR. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. The Journal of clinical investigation. 2013;123:5310–5318. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, Mueller C, Messina LM, Wilson JM, Brantly M, Knop DR, Ye GJ, Chulay JD. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Human gene therapy. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Taylor LE, Flanigan KM, Gastier-Foster JM, Astbury C, Kota J, Sahenk Z, Walker CM, Clark KR. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Annals of neurology. 2010;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J, Petry H, Gaudet D. Immune Responses to Intramuscular Administration of Alipogene Tiparvovec (AAV1-LPL(S447X)) in a Phase II Clinical Trial of Lipoprotein Lipase Deficiency Gene Therapy. Human gene therapy. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller C, Braag SA, Martino AT, Tang Q, Campbell-Thompson M, Flotte TR. The pros and cons of immunomodulatory IL-10 gene therapy with recombinant AAV in a Cftr−/−-dependent allergy mouse model. Gene therapy. 2009;16:172–183. doi: 10.1038/gt.2008.156. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Pattanayak A, Song M, Kou J, Taguchi H, Paul S, Ponnazhagan S, Lalonde R, Fukuchi K. Muscle-directed anti-Abeta single-chain antibody delivery via AAV1 reduces cerebral Abeta load in an Alzheimer's disease mouse model. Journal of molecular neuroscience. 2013;49:277–288. doi: 10.1007/s12031-012-9877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, Bennett J. The human visual cortex responds to gene therapy-mediated recovery of retinal function. The Journal of clinical investigation. 2011;121:2160–2168. doi: 10.1172/JCI57377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Molecular therapy. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benraiss A, Toner MJ, Xu Q, Bruel-Jungerman E, Rogers EH, Wang F, Economides AN, Davidson BL, Kageyama R, Nedergaard M, Goldman SA. Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington's disease. Cell stem cell. 2013;12:787–799. doi: 10.1016/j.stem.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV, Cheng SH, Shihabuddin LS, Kaspar BK. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Molecular therapy. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lheriteau E, Petit L, Weber M, Le Meur G, Deschamps JY, Libeau L, Mendes-Madeira A, Guihal C, Francois A, Guyon R, Provost N, Lemoine F, Papal S, El-Amraoui A, Colle MA, Moullier P, Rolling F. Successful gene therapy in the RPGRIP1-deficient dog: a large model of cone-rod dystrophy. Molecular therapy. 2014;22:265–277. doi: 10.1038/mt.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popiel HA, Takeuchi T, Fujita H, Yamamoto K, Ito C, Yamane H, Muramatsu S, Toda T, Wada K, Nagai Y. Hsp40 gene therapy exerts therapeutic effects on polyglutamine disease mice via a non-cell autonomous mechanism. PloS one. 2012;7:e51069. doi: 10.1371/journal.pone.0051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber C, Neacsu I, Krautz B, Schlegel P, Sauer S, Raake P, Ritterhoff J, Jungmann A, Remppis AB, Stangassinger M, Koch WJ, Katus HA, Muller OJ, Most P, Pleger ST. Therapeutic safety of high myocardial expression levels of the molecular inotrope S100A1 in a preclinical heart failure model. Gene therapy. 2014;21:131–138. doi: 10.1038/gt.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JM, Navarro S, Doki T, Stewart JM, Mitsuhashi N, Kearns-Jonker M. Gene transfer of heme oxygenase-1 using an adeno-associated virus serotype 6 vector prolongs cardiac allograft survival. Journal of transplantation. 2012;2012:740653. doi: 10.1155/2012/740653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo X, Hall G, Li S, Bird A, Lavin PJ, Winn MP, Kemper AR, Brown TT, Koeberl DD. Hepatorenal correction in murine glycogen storage disease type I with a double-stranded adeno-associated virus vector. Molecular therapy. 2011;19:1961–1970. doi: 10.1038/mt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. The New England journal of medicine. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anisimov A, Alitalo A, Korpisalo P, Soronen J, Kaijalainen S, Leppanen VM, Jeltsch M, Yla-Herttuala S, Alitalo K. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circulation research. 2009;104:1302–1312. doi: 10.1161/CIRCRESAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kou J, Yang J, Lim JE, Pattanayak A, Song M, Planque S, Paul S, Fukuchi KI. Catalytic Immunoglobulin Gene Delivery in a Mouse Model of Alzheimer's Disease: Prophylactic and Therapeutic Applications. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaanine AH, Nonnenmacher M, Kohlbrenner E, Jin D, Kovacic JC, Akar FG, Hajjar RJ, Weber T. Effect of bortezomib on the efficacy of AAV9.SERCA2a treatment to preserve cardiac function in a rat pressure-overload model of heart failure. Gene therapy. 2014;21:379–386. doi: 10.1038/gt.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M, Zentilin L, Thum T, Laggerbauer B, Engelhardt S. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127:2097–2106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 54.Timiri Shanmugam PS, Dayton RD, Palaniyandi S, Abreo F, Caldito G, Klein RL, Sunavala-Dossabhoy G. Recombinant AAV9-TLK1B administration ameliorates fractionated radiation-induced xerostomia. Human gene therapy. 2013;24:604–612. doi: 10.1089/hum.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perdomini M, Belbellaa B, Monassier L, Reutenauer L, Messaddeq N, Cartier N, Crystal RG, Aubourg P, Puccio H. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nature medicine. 2014 doi: 10.1038/nm.3510. [DOI] [PubMed] [Google Scholar]
- 56.Sondhi D, Scott EC, Chen A, Hackett NR, Wong AM, Kubiak A, Nelvagal HR, Pearse Y, Cotman SL, Cooper JD, Crystal RG. Partial Correction of the CNS Lysosomal Storage Defect in a Mouse Model of Juvenile Neuronal Ceroid Lipofuscinosis by Neonatal CNS Administration of an Adeno-Associated Virus Serotype rh.10 Vector Expressing the Human CLN3 Gene. Human gene therapy. 2014;25:223–239. doi: 10.1089/hum.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kojima Y, Honda K, Hamada H, Kobayashi N. Oncolytic gene therapy combined with double suicide genes for human bile duct cancer in nude mouse models. The Journal of surgical research. 2009;157:e63–e70. doi: 10.1016/j.jss.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Pan JG, Zhou X, Luo R, Han RF. The adeno-associated virus-mediated HSV-TK/GCV suicide system: a potential strategy for the treatment of bladder carcinoma. Medical oncology. 2012;29:1938–1947. doi: 10.1007/s12032-011-0091-x. [DOI] [PubMed] [Google Scholar]
- 59.Kim JY, Kim JH, Khim M, Lee HS, Jung JH, Moon DH, Jeong S, Lee H. Persistent anti-tumor effects via recombinant adeno-associated virus encoding herpes thymidine kinase gene monitored by PET-imaging. Oncology reports. 2011;25:1263–1269. doi: 10.3892/or.2011.1190. [DOI] [PubMed] [Google Scholar]
- 60.Trepel M, Stoneham CA, Eleftherohorinou H, Mazarakis ND, Pasqualini R, Arap W, Hajitou A. A heterotypic bystander effect for tumor cell killing after adeno-associated virus/phage-mediated, vascular-targeted suicide gene transfer. Molecular cancer therapeutics. 2009;8:2383–2391. doi: 10.1158/1535-7163.MCT-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denny WA. Prodrugs for Gene-Directed Enzyme-Prodrug Therapy (Suicide Gene Therapy) Journal of biomedicine & biotechnology. 2003;2003:48–70. doi: 10.1155/S1110724303209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y, Pu PY. The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer gene therapy. 2010;17:192–202. doi: 10.1038/cgt.2009.64. [DOI] [PubMed] [Google Scholar]
- 63.Sanson M, Marcaud V, Robin E, Valery C, Sturtz F, Zalc B. Connexin 43-mediated bystander effect in two rat glioma cell models. Cancer gene therapy. 2002;9:149–155. doi: 10.1038/sj.cgt.7700411. [DOI] [PubMed] [Google Scholar]
- 64.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer research. 2007;67:1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 66.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 67.Lu L, Luo ST, Shi HS, Li M, Zhang HL, He SS, Liu Y, Pan Y, Yang L. AAV2-mediated gene transfer of VEGF-Trap with potent suppression of primary breast tumor growth and spontaneous pulmonary metastases by long-term expression. Oncology reports. 2012;28:1332–1338. doi: 10.3892/or.2012.1915. [DOI] [PubMed] [Google Scholar]
- 68.Wu QJ, Gong CY, Luo ST, Zhang DM, Zhang S, Shi HS, Lu L, Yan HX, He SS, Li DD, Yang L, Zhao X, Wei YQ. AAV-mediated human PEDF inhibits tumor growth and metastasis in murine colorectal peritoneal carcinomatosis model. BMC cancer. 2012;12:129. doi: 10.1186/1471-2407-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He SS, Shi HS, Yin T, Li YX, Luo ST, Wu QJ, Lu L, Wei YQ, Yang L. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in mice. Oncology reports. 2012;27:1142–1148. doi: 10.3892/or.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bui Nguyen TM, Subramanian IV, Xiao X, Nguyen P, Ramakrishnan S. Adeno-associated virus-mediated delivery of kringle 5 of human plasminogen inhibits orthotopic growth of ovarian cancer. Gene therapy. 2010;17:606–615. doi: 10.1038/gt.2010.15. [DOI] [PubMed] [Google Scholar]
- 71.Tse LY, Sun X, Jiang H, Dong X, Fung PW, Farzaneh F, Xu R. Adeno-associated virus-mediated expression of kallistatin suppresses local and remote hepatocellular carcinomas. The journal of gene medicine. 2008;10:508–517. doi: 10.1002/jgm.1180. [DOI] [PubMed] [Google Scholar]
- 72.Buhles A, Collins SA, van Pijkeren JP, Rajendran S, Miles M, O'Sullivan GC, O'Hanlon DM, Tangney M. Anti-metastatic effects of viral and non-viral mediated Nk4 delivery to tumours. Genetic vaccines and therapy. 2009;7:5. doi: 10.1186/1479-0556-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahadevan M, Liu Y, You C, Luo R, You H, Mehta JL, Hermonat PL. Generation of robust cytotoxic T lymphocytes against prostate specific antigen by transduction of dendritic cells using protein and recombinant adeno-associated virus. Cancer immunology, immunotherapy. 2007;56:1615–1624. doi: 10.1007/s00262-007-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiu TL, Peng CW, Wang MJ. Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncology reports. 2011;25:1373–1380. doi: 10.3892/or.2011.1213. [DOI] [PubMed] [Google Scholar]
- 75.Yiang GT, Harn HJ, Yu YL, Hu SC, Hung YT, Hsieh CJ, Lin SZ, Wei CW. Immunotherapy: rAAV2 expressing interleukin-15 inhibits HeLa cell tumor growth in mice. Journal of biomedical science. 2009;16:47. doi: 10.1186/1423-0127-16-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yiang GT, Chou PL, Tsai HF, Chen LA, Chang WJ, Yu YL, Wei CW. Immunotherapy for SV40 T/t antigen-induced breast cancer by recombinant adeno-associated virus serotype 2 carrying interleukin-15 in mice. International journal of molecular medicine. 2012;29:809–814. doi: 10.3892/ijmm.2012.921. [DOI] [PubMed] [Google Scholar]
- 77.Han T, Abdel-Motal UM, Chang DK, Sui J, Muvaffak A, Campbell J, Zhu Q, Kupper TS, Marasco WA. Human anti-CCR4 minibody gene transfer for the treatment of cutaneous T-cell lymphoma. PloS one. 2012;7:e44455. doi: 10.1371/journal.pone.0044455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meijer DH, Maguire CA, LeRoy SG, Sena-Esteves M. Controlling brain tumor growth by intraventricular administration of an AAV vector encoding IFN-beta. Cancer gene therapy. 2009;16:664–671. doi: 10.1038/cgt.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Lu J, He ML, Li Z, Zhang B, Zhou LH, Li Q, Li G, Wang L, Tian WD, Peng Y, Li XP. Antitumor effects of interferon-alpha on cell growth and metastasis in human nasopharyngeal carcinoma. Current cancer drug targets. 2012;12:561–570. doi: 10.2174/156800912800673293. [DOI] [PubMed] [Google Scholar]
- 80.Nieto K, Kern A, Leuchs B, Gissmann L, Muller M, Kleinschmidt JA. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antiviral therapy. 2009;14:1125–1137. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- 81.Pan J, Zhang Q, Zhou J, Ma D, Xiao X, Wang DW. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Molecular cancer therapeutics. 2009;8:2754–2761. doi: 10.1158/1535-7163.MCT-08-1176. [DOI] [PubMed] [Google Scholar]
- 82.Li R, Hu H, Ma H, Chen L, Zhou B, Liu Y, Liang C. The anti-tumor effect and increased tregs infiltration mediated by rAAV-SLC vector. Molecular biology reports. 2013;40:5615–5623. doi: 10.1007/s11033-013-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elhag OA, Hu XJ, Wen-Ying Z, Li X, Yuan YZ, Deng LF, Liu DL, Liu YL, Hui G. Reconstructed adeno-associated virus with the extracellular domain of murine PD-1 induces antitumor immunity. Asian Pacific journal of cancer prevention. 2012;13:4031–4036. doi: 10.7314/apjcp.2012.13.8.4031. [DOI] [PubMed] [Google Scholar]
- 84.Sun H, Liu Y, Bu D, Liu X, Norris JS, Xiao S. Efficient growth suppression and apoptosis in human laryngeal carcinoma cell line HEP-2 induced by an adeno-associated virus expressing human FAS ligand. Head & neck. 2012;34:1628–1633. doi: 10.1002/hed.21985. [DOI] [PubMed] [Google Scholar]
- 85.He ZH, Lei Z, Zhen Y, Gong W, Huang B, Yuan Y, Zhang GM, Wang XJ, Feng ZH. Adeno-associated virus-mediated expression of recombinant CBD-HepII polypeptide of human fibronectin inhibits metastasis of breast cancer. Breast cancer research and treatment. 2014;143:33–45. doi: 10.1007/s10549-013-2783-8. [DOI] [PubMed] [Google Scholar]
- 86.Qin TJ, Ma W, Liu SX, Yang GX, Wang QY, Zhao XH. Secretory expression of Par-4 SAC-HA2TAT following adeno-associated virus-mediated gene transfer induces apoptosis in HepG2 cells. Molecular medicine reports. 2010;3:749–757. doi: 10.3892/mmr.2010.343. [DOI] [PubMed] [Google Scholar]
- 87.Lv F, Qiu Y, Zhang Y, Liu S, Shi J, Liu Y, Zheng D. Adeno-associated virus-mediated anti-DR5 chimeric antibody expression suppresses human tumor growth in nude mice. Cancer letters. 2011;302:119–127. doi: 10.1016/j.canlet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Xiaojiang T, Jinsong Z, Jiansheng W, Chengen P, Guangxiao Y, Quanying W. Adeno-associated virus harboring fusion gene NT4-ant-shepherdin induce cell death in human lung cancer cells. Cancer investigation. 2010;28:465–471. doi: 10.3109/07357900903095706. [DOI] [PubMed] [Google Scholar]
- 89.Ng SS, Gao Y, Chau DH, Li GH, Lai LH, Huang PT, Huang CF, Huang JJ, Chen YC, Kung HF, Lin MC. A novel glioblastoma cancer gene therapy using AAV-mediated long-term expression of human TERT C-terminal polypeptide. Cancer gene therapy. 2007;14:561–572. doi: 10.1038/sj.cgt.7701038. [DOI] [PubMed] [Google Scholar]
- 90.Sun A, Tang J, Terranova PF, Zhang X, Thrasher JB, Li B. Adeno-associated virus-delivered short hairpin-structured RNA for androgen receptor gene silencing induces tumor eradication of prostate cancer xenografts in nude mice: a preclinical study. International journal of cancer. Journal international du cancer. 2010;126:764–774. doi: 10.1002/ijc.24778. [DOI] [PubMed] [Google Scholar]
- 91.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Liu X, Chen X, Guo Z, Liu J, Hao J, Zhang J. Real-time monitoring of miRNA function in pancreatic cell lines using recombinant AAV-based miRNA Asensors. PloS one. 2013;8:e66315. doi: 10.1371/journal.pone.0066315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruan Q, Xi L, Boye SL, Han S, Chen ZJ, Hauswirth WW, Lewin AS, Boulton ME, Law BK, Jiang WG, Jiang H, Cai J. Development of an anti-angiogenic therapeutic model combining scAAV2-delivered siRNAs and noninvasive photoacoustic imaging of tumor vasculature development. Cancer letters. 2013;332:120–129. doi: 10.1016/j.canlet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang K, Jiao X, Liu X, Zhang B, Wang J, Wang Q, Tao Y, Zhang D. Knockdown of snail sensitizes pancreatic cancer cells to chemotherapeutic agents and irradiation. International journal of molecular sciences. 2010;11:4891–4892. doi: 10.3390/ijms11124891. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life sciences. 2008;82:1154–1161. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Huang F, Cai H, Zhong S, Liu X, Tan WS. Potent antitumor effect of TRAIL mediated by a novel adeno-associated viral vector targeting to telomerase activity for human hepatocellular carcinoma. The journal of gene medicine. 2008;10:518–526. doi: 10.1002/jgm.1177. [DOI] [PubMed] [Google Scholar]
- 97.He LF, Wang YG, Xiao T, Zhang KJ, Li GC, Gu JF, Chu L, Tang WH, Tan WS, Liu XY. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-beta expression driven by hTERT promoter. Cancer letters. 2009;286:196–205. doi: 10.1016/j.canlet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 98.Ma H, Liu Y, Liu S, Xu R, Zheng D. Oral adeno-associated virus-sTRAIL gene therapy suppresses human hepatocellular carcinoma growth in mice. Hepatology. 2005;42:1355–1363. doi: 10.1002/hep.20918. [DOI] [PubMed] [Google Scholar]
- 99.Lee HS, Kim JY, Lee WI, Kim SJ, Ko MJ, Jeong S, Park K, Choe H, Lee H. Acquisition of selective antitumoral effects of recombinant adeno-associated virus by genetically inserting tumor-targeting peptides into capsid proteins. Oncology letters. 2011;2:1113–1119. doi: 10.3892/ol.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan L, Zhao H, Zhang L, Liu X. The efficacy of combination therapy using adeno-associated virus-mediated co-expression of apoptin and interleukin-24 on hepatocellular carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:3027–3034. doi: 10.1007/s13277-013-0867-z. [DOI] [PubMed] [Google Scholar]
- 101.Pan JG, Zhou X, Zeng GW, Han RF. Potent antitumour activity of the combination of HSV-TK and endostatin armed oncolytic adeno-associated virus for bladder cancer in vitro and in vivo. Journal of surgical oncology. 2012;105:249–257. doi: 10.1002/jso.22107. [DOI] [PubMed] [Google Scholar]
- 102.Pan JG, Luo RQ, Zhou X, Han RF, Zeng GW. Potent antitumor activity of the combination of HSV-TK and endostatin by adeno-associated virus vector for bladder cancer in vivo. Clinical laboratory. 2013;59:1147–1158. doi: 10.7754/clin.lab.2012.121109. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Huang F, Cai H, Wu Y, He G, Tan WS. The efficacy of combination therapy using adeno-associated virus-TRAIL targeting to telomerase activity and cisplatin in a mice model of hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2010;136:1827–1837. doi: 10.1007/s00432-010-0841-8. [DOI] [PubMed] [Google Scholar]
- 104.Jiang M, Liu Z, Xiang Y, Ma H, Liu S, Liu Y, Zheng D. Synergistic antitumor effect of AAV-mediated TRAIL expression combined with cisplatin on head and neck squamous cell carcinoma. BMC cancer. 2011;11:54. doi: 10.1186/1471-2407-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Subramanian IV, Devineni S, Ghebre R, Ghosh G, Joshi HP, Jing Y, Truskinovsky AM, Ramakrishnan S. AAV-P125A-endostatin and paclitaxel treatment increases endoreduplication in endothelial cells and inhibits metastasis of breast cancer. Gene therapy. 2011;18:145–154. doi: 10.1038/gt.2010.118. [DOI] [PubMed] [Google Scholar]
- 106.Subramanian IV, Bui Nguyen TM, Truskinovsky AM, Tolar J, Blazar BR, Ramakrishnan S. Adeno-associated virus-mediated delivery of a mutant endostatin in combination with carboplatin treatment inhibits orthotopic growth of ovarian cancer and improves long-term survival. Cancer research. 2006;66:4319–4328. doi: 10.1158/0008-5472.CAN-05-3297. [DOI] [PubMed] [Google Scholar]
- 107.Hamner JB, Sims TL, Cutshaw A, Dickson PV, Rosati S, McGee M, Ng CY, Davidoff AM. The efficacy of combination therapy using adeno-associated virus--interferon beta and trichostatin A in vitro and in a murine model of neuroblastoma. Journal of pediatric surgery. 2008;43:177–182. doi: 10.1016/j.jpedsurg.2007.09.048. discussion 182-173. [DOI] [PubMed] [Google Scholar]
- 108.Xue Z, Sun PH, Zhu LM, Jiang SH, Qiao MM, Chi AL, Tu SP. Adeno-associated virus-mediated survivin mutant Thr34Ala cooperates with oxaliplatin to inhibit tumor growth and angiogenesis in colon cancer. Oncology reports. 2011;25:1039–1046. doi: 10.3892/or.2011.1166. [DOI] [PubMed] [Google Scholar]
- 109.Kanazawa T, Mizukami H, Okada T, Hanazono Y, Kume A, Nishino H, Takeuchi K, Kitamura K, Ichimura K, Ozawa K. Suicide gene therapy using AAV-HSVtk/ganciclovir in combination with irradiation results in regression of human head and neck cancer xenografts in nude mice. Gene therapy. 2003;10:51–58. doi: 10.1038/sj.gt.3301837. [DOI] [PubMed] [Google Scholar]
- 110.Zhou SJ, Li SW, Wang JJ, Liu ZJ, Yin GB, Gong JP, Liu CA. High-intensity focused ultrasound combined with herpes simplex virus thymidine kinase gene-loaded ultrasound-targeted microbubbles improved the survival of rabbits with VX(2) liver tumor. The journal of gene medicine. 2012;14:570–579. doi: 10.1002/jgm.2668. [DOI] [PubMed] [Google Scholar]
- 111.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nature genetics. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 112.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature medicine. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 113.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 114.Buchlis G, Podsakoff GM, Radu A, Hawk SM, Flake AW, Mingozzi F, High KA. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119:3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, Rouhani F, Conlon TJ, Calcedo R, Betts MR, Spencer C, Byrne BJ, Wilson JM, Flotte TR. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet neurology. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 117.Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80:1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Human gene therapy. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Archives of ophthalmology. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di L, Zhu Y, Jia J, Yu J, Song G, Zhang J, Che L, Yang H, Han Y, Ma B, Zhang C, Yuan Y, You M, Wan F, Wang X, Zhou X, Ren J. Clinical safety of induced CTL infusion through recombinant adeno-associated virus-transfected dendritic cell vaccination in Chinese cancer patients. Clinical & translational oncology. 2012;14:675–681. doi: 10.1007/s12094-012-0854-7. [DOI] [PubMed] [Google Scholar]
- 121.Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, Furst DE, Anklesaria P, Heald AE. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Annals of the rheumatic diseases. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- 122.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. Journal of virology. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]