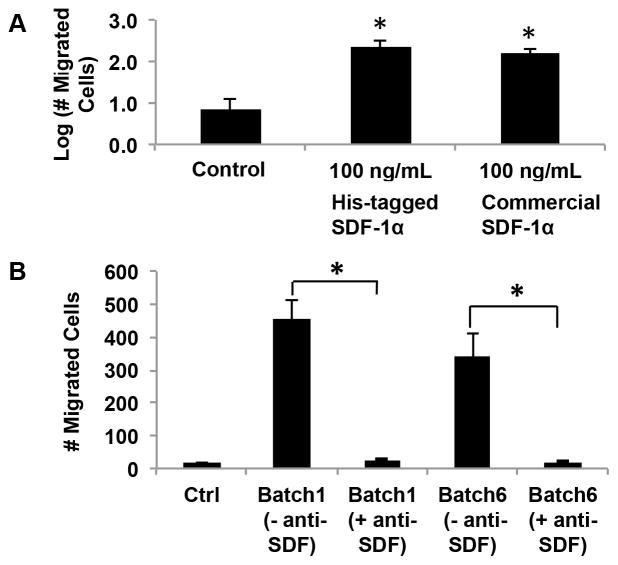
Figure 2. In-house produced recombinant His-tagged SDF-1α is bioactive.
(A) The bioactivity of His-tagged SDF-1α was verified via a trans-well migration assay, in which commercially available SDF-1α, His-tagged SDF-1α, or no chemokine control conditions were maintained in the lower chamber, whereas migrating cells were placed in the upper chamber separated by a thin porous membrane. The number of cells that crossed the membrane was quantified. A statistically significant increase in trans-migration of cells over the control case was observed for both in-house produced His-tagged SDF-1α and commercially purchased SDF-1α, and the latter two were not statistically distinguishable (One-way ANOVA, *P<0.05). (B) The specificity of His-tagged SDF-1α was confirmed, via an antibody neutralization assay, in which migration was tested with SDF-1α in the lower chamber that was equilibrated with or without anti-SDF antibody. Excess antibody neutralization (Ab:SDF-1α of 14:1) of two recombinant batches showed complete abolishment of chemotactic behavior to levels comparable to the control case (no chemokine).