Abstract
INTRODUCTION
Performance status (PS) is the only known clinical predictor of outcome in patients with advanced non-small-cell lung cancer (NSCLC), although pharmacogenomic markers may also correlate with outcome. The aim of our study was to correlate clinical and pharmacogenomic measures with overall survival.
METHODS
This was an IRB approved, retrospective study in which the medical records of 50 patients with advanced NSCLC from 1998–2008 were reviewed, and gender, race, PS, and chemotherapy regimens were documented. Stromal expression of pharmacogenomic markers (VEGFR, ERCC1, 14-3-3σ, pAKT, and PTEN) was measured. Clinical factors and pharmacogenomics markers were compared to overall survival using a Cox proportional hazards model.
RESULTS
Forty patients received platinum-based therapy. Median age was 65 years. Improved PS, female gender, and gemcitabine therapy were significantly associated with longer overall survival (P = 0.004, P = 0.04, and P = 0.003, respectively). Age was not associated with survival. Caucasians had better overall survival in comparison to African Americans with median survival of 14.8 months versus 10.4 months (P = 0.1). Patients treated with platinum-based therapy had better survival of 15 months versus 8 months for non-platinum based therapy (P = 0.01). There was no significant association between any of the pharmacogenomics markers and overall survival other than in patients treated with platinum, in whom ERCC1 negativity was strongly associated with longer survival (P = 0.007).
CONCLUSION
ERCC1 negativity with platinum therapy, gemcitabine therapy, good PS, and female gender all correlated with improved overall survival in patients with advanced NSCLC.
Keywords: pharmacogenomics, selection of therapy, non-small-cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States. Even after 30 years of improved therapeutic approaches, the 5-year mortality of lung cancer remains alarmingly high at 86%.1 The emerging application of pharmacogenomics offers the potential to identify drugs and drug combinations in the treatment of lung cancer according to an individual’s unique genetic makeup. A series of randomized clinical trials have demonstrated that survival of patients with stage III or IV non-small-cell lung cancer (NSCLC) is improved with the use of chemotherapy compared to best supportive care.2,3 Among the initial chemotherapeutic agents that demonstrated reproducible activity in NSCLC was the non-classical alkylating agent cisplatin. More recently, newer agents such as gemcitabine and taxanes have been combined with cisplatin; however, the ability to predict which patients are likely to benefit from chemotherapy remains a challenging dilemma with no uniformly reliable clinical predictors other than performance status (PS) and weight loss.
Several studies have linked cisplatin resistance to high expression of the DNA repair gene excision repair cross-complementation group 1 (ERCC1).4–7 ERCC1 is 1 of 16 genes that encodes the nucleotide excision repair complex. ERCC1 is a nucleotide excision repair enzyme playing an important role in DNA repair mechanism that removes the therapeutic platinum–DNA adducts from the tumor DNA. NSCLC patients with surgically removed early stage tumors, who received no further therapy, had a better survival if their tumors were ERCC1 positive.8 These studies also have emphasized that patients with lowest levels of mRNA expression of ERCC1 tend to have better response to cisplatin-based therapy. This indicates the importance of considering ERCC1 mRNA expression as a predictive marker for the effectiveness of cisplatin-based chemotherapy.
In addition to ERCC1, there are several other pharmacogenomic markers that could have prognostic impact, although they act through different mechanisms. Angiogenesis plays an important role in tumor growth and has attracted interest as a potential therapeutic target.9 Studies have shown that 14-3-3 sigma (14-3-3σ) protein levels are more highly expressed in human lung cancers compared to normal tissues.
The aim of this study was to correlate clinical factors and pharmacogenomic markers, such as ERCC1 and 14-3-3σ, with overall survival in patients with advanced stage NSCLC and also to determine the feasibility of utilizing pharmacogenomic markers as prognostic markers and to determine their value for incorporation into future treatment protocols.
Materials and Methods
Fifty patients with advanced stage NSCLC were included in the study in the period between January 2007 and December 2008. We included patients with advanced stage NSCLC (recurrent NSCLC or stage III or IV NSCLC; squamous, adenocarcinoma, or large cell undifferentiated NSCLC) with adequate tissue to undergo further analysis. Most of the patients with advanced stage were only diagnosed with fine needle aspiration or small biopsies. Three patients had surgical resection, followed by adjuvant systemic chemotherapy, however, were included in the study as they became progressive with recurrent advanced NSCLC. Pharmacogenomic testing was carried out in the majority of the 50 patients using the biopsy samples of the advanced disease, but for patients who had prior surgical resection, the available surgically resected tumor was tested. Five patients received radiation for limited periods of time.
All patients had chest X-ray and a computed tomography (CT) scan of the chest and upper abdomen before entry into the study and underwent repeated evaluation at least every 6 weeks. All patients gave signed informed consent, and the study was approved by the institutional ethics review boards. Archival tumor specimens from each patient were retrieved from the pathology core facility after review of the H&E (Hematoxylin and eosin) stained slides.
This was a retrospective study, approved by the University of Cincinnati Institutional Review Board, in which 50 patients with stages III and IV NSCLC from the University Medical Center and the VA medical center were included between 1998 until 2008. The patients’ medical records were reviewed and their gender, race, PS, chemotherapy regimens, and pharmacogenomic marker levels were documented and evaluated for correlation with survival. Pharmacogenomic markers were measured by pathologists at the University of Cincinnati. The evaluation was blinded and they used immunohistochemical (IHC) staining and included: ERCC1, vascular endothelial growth factors (VEGF) and VEGF receptor, nuclear factor kappa beta (NF-kB [p65]), 14-3-3σ, and phosphorylated AKT (pAKT) and PTEN.
Immunohistochemical staining
IHC staining was performed using Vectastain Elite ABC universal staining kit (Vector Laboratories, CA, USA) according to the manufacturer’s protocol. Paraffin blocks of stage III and IV NSCLC were sectioned at 4 microseconds and de-waxed for 2 hours at 65 °C. Sections were boiled at 95 °C in antigen demasking buffer (10 mM sodium citrate at pH 6.0) for 15 minutes. These sections were further incubated with 3% H2O2 in 100% methanol to quench the endogenous peroxidase activity and blocked with 10% goat or horse serum for 2 hours. The treated sections were incubated overnight at 4 °C with anti-VEGF (A-20) rabbit polyclonal at 1:100 dilution; anti-p65 (C-20) rabbit polyclonal at 1:100 dilution; anti-14-3-3σ (N-14) goat polyclonal at 1:500 dilution (Santa Cruz Biotechnology, CA, USA); anti-VEGFR2 (KDR-55B11) rabbit monoclonal at 1:250 dilution; anti-pAKT (ser 473) rabbit monoclonal at 1:100 dilution (Cell Signaling, MA, USA); anti-PTEN rabbit polyclonal at 1:100 dilution (Abcam, MA, USA); and anti-ERCC1 (8F1) rabbit polyclonal at 1:200 dilution (Thermo Fisher Scientific, CA, USA). Peroxidase staining was revealed using a DAB-enhanced liquid substrate system (3,3′-diaminobenzidine tetrahydrochloride) (Sigma, MO, USA). Stained sections were counterstained using Mayers hematoxylin. Results were interpreted independently by two pathologists and graded by the following criteria: Grade 0 (negative or non-reactive expression), Grade 1 (weak or focal expression), Grade 2 (moderate expression), and Grade 3 (strong or diffused expression). Grades ≤5% were considered negative. All tumor samples were graded using normal samples as a control. Figures 1A and 1B demonstrate ERCC1-positive and -negative expressions, respectively.
Figure 1.
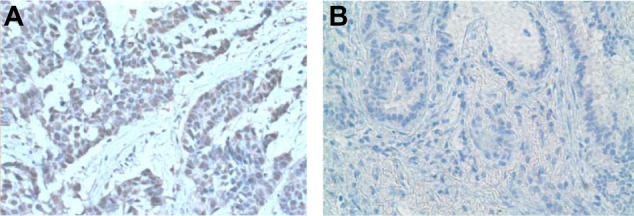
(A) ERCC 1 overexpression. (B) Negative ERCC 1 expression.
Statistical Analysis
The main objective of this study was to identify molecular determinants of clinical outcomes related to cancer prognosis and survival. The primary outcomes were overall survival and progression-free survival.
The expression rates of the protein markers VEGF receptor and 14-3-3σ, as determined by IHC labeling, were treated as continuous variables, while the remaining markers (VEGF, P65, PTEN, pAKT, and ERCC1) were treated as ordinal categorical variables. Cells with protein expression rates above 0 (VEGF receptor and 14-3-3σ) or stained as 1+ or 2+ (VEGF, P65, PTEN, pAKT, and ERCC1) were considered positive. Cells with 0 expression or stained as 0+ were considered negative for the marker.
Overall survival was defined as the time from the date of diagnosis to the time of death from any cause or the time at the last follow-up. Progression-free survival was defined as the duration between the date of diagnosis and disease progression or the last follow-up.
The Kaplan–Meier method was used to estimate the distribution of survival time of the patients, overall and after stratifying by race and gender. Univariate analysis was conducted to examine the survival curves across the protein expression levels of the molecular markers (classified as positive and negative) using the log rank test.
In order to determine the joint effect of the predictors (age, gender, race, type of chemotherapy, and protein expression of each marker) on the overall survival and progression-free survival of the patients, a Cox regression model was used. Covariates were chosen using the backward selection technique at the 10% significance level.
The association between the molecular markers and demographic and clinical parameters was examined. Comparisons of categorical data were performed using the Chi-square test or Fisher’s exact test. The Wilcoxon two-sample test with t approximation was used to compare continuous data that were not normally distributed. Continuous variables, including the overall survival and progression-free survival, were correlated with expression levels of the markers using Spearman correlation coefficients. A two-tailed P value ≤0.1 was considered significant in this preliminary analysis.
Results
Fifty patients with histologically proven NSCLC of advanced stage (stage III or IV) were included in our study. Forty patients received a platinum-based chemotherapy regimen, while 10 received non-platinum based regimens. Median age was 65 years (range 42–84); age did not correlate with OS (Overall Survival) (P = 0.24). ECOG PS was 0 in 8 patients (16%), 1 in 29 patients (58%), 2 in 7 patients (14%), and 3 in 6 patients (14%). ECOG PS of 0 and 1 were significantly associated with higher overall survival (P = 0.004). Females had significantly longer survival compared to males (31.7 months versus 12.4 months, P = 0.04) as shown in Figure 2 and Table 1. Caucasians had better overall survival in comparison to African Americans with median survival of 14.8 months versus 10.4 months, although this did not reach statistical significance (P = 0.1) as shown in Table 1.
Figure 2.
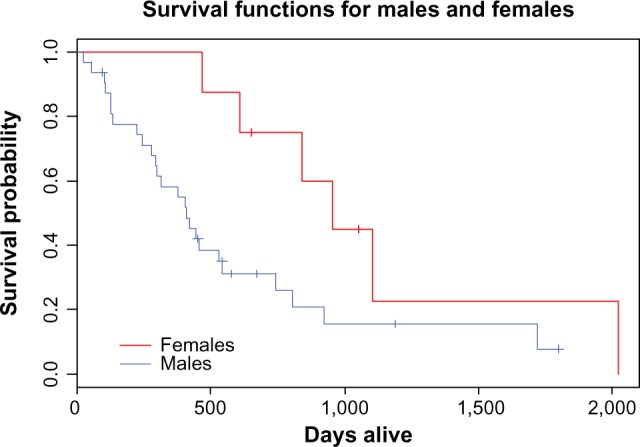
Overall survival in our studied male and female patients.
Table 1.
Clinical variants and pharmacogenomics markers affecting outcome of NSCLC.
| VARIANT | MEDIAN SURVIVAL | P VALUE |
|---|---|---|
| -Gender (n = 50) Females (n = 8) Males (n = 42) |
31.7 months 12.5 months |
0.04 |
| -Race (n = 50) Caucasians (n = 45) African Americans (n = 5) |
14.8 months 10.4 months |
0.1 |
| -Performance status (n = 50) 0, 1 (n = 37) 2, 3 (n = 13) |
15 months 6.3 months |
0.007 |
| -Gemcitabine based therapy (n = 12) -Non-Gemcitabine therapy (n = 38) |
19 months 13 months |
0.003 |
| -Platinum based therapy (n = 40) -Non-platinum based therapy (n = 10) |
15.3 months 8.4 months |
0.1 |
| -ERCC1 in platinum treated patients (n = 40) ERCC1 negative (n = 14) ERCC1 positive (n = 12) ERCC1 strongly positive (n = 14) |
36 months 15 months 12 months |
0.035 0.007 |
| 14-3-3 sigma (n = 48) -High expression (n = 7) -Low expression (n = 41) |
13.5 months 13 months |
Not significant |
| -VEGFR expression (n = 49) -VEGFR high expression (n = 26) -VEGFR low expression (n = 23) |
14 months 9.7 months |
0.1 |
| -Platinum treated ERCC1 negative with PS 0,1 (n = 11) | 22.4 months | |
| -Platinum treated ERCC1 negative with PS 2,3 (n = 2) | N/A | |
| Platinum treated ERCC1 positive with PS 0, 1 (n = 23) | 16.8 months | |
| -Platinum treated ERCC1 positive with PS 2,3 (n = 4) | 14.8 months | |
| Non-platinum treated ERCC1 positive with PS 0,1 (n = 1) | N/A | |
| Non-platinum treated ERCC1 negative with PS 0,1 (n = 4) | 7.6 | |
| Non-platinum treated ERCC1 positive and PS 0, 1 (n = 2) | N/A | |
| Non-platinum treated ERCC1 positive and PS 2,3 (n = 3) | 6.3 | |
| pAKT positive (n = 13) | 13.5 months | |
| pAKT negative (n = 33) | 13 months | |
| PTEN positive (n = 31) | 13.4 months | |
| PTEN negative (n = 15) | 13 months | |
| P65 positive (n = 4) | 9.9 months | 0.1 |
| P65 negative (n = 42) | 13 months |
Patients treated with gemcitabine therapy had a median survival of 19 months versus 13 months in the non-gemcitabine therapy group (P = 0.003); patients treated with platinum-based therapy had a median survival of 15 months versus 8 months in the non-platinum based therapy group, but this did not reach statistical significance. However, in patients treated with platinum, ERCC1 negativity was strongly associated with longer survival (P = 0.007) as shown in Figure 3.
Figure 3.
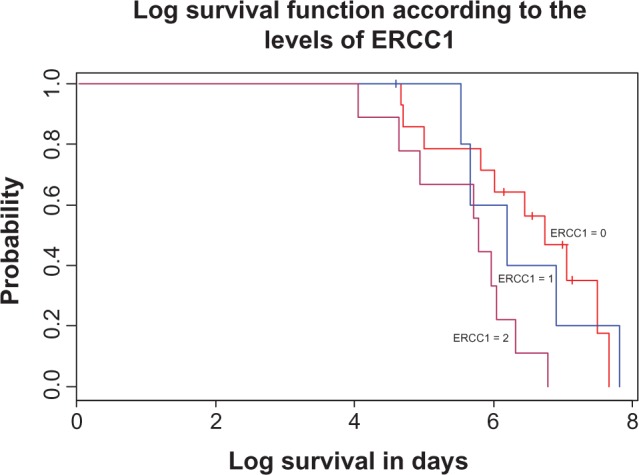
Correlation between ERCC1 levels and overall survival in patients with NSCLC treated with standard therapy.
Higher expression of the VEGF receptor was associated with improved overall survival of 14 months versus 9.7 months with lower expression, but the difference did not reach statistical significance. 14-3-3σ, pAKT, and PTEN tissue expression did not correlate with overall survival.
NF-kB (p65) expression was positive in 4 out of 46 patients’ lung cancer tissue. The positivity of NF-kB (p65) was associated with shorter survival of 9.9 months versus 13 months in patients with negative expression of the same receptor, with only a statistical trend of P = 0.1 (see Table 1).
Discussion
Since cisplatin and carboplatin are the most commonly used base agents for combination chemotherapy for the treatment of advanced NSCLC, many studies have focused on the benefit of platinum doublets. Older randomized trials repeatedly failed to identify differences in survival between any of these doublets, which gave way to the new era of personalized chemotherapy and the selection of patients with ERCC1 negative expression for receiving platinum-based chemotherapy. Adjuvant therapy with platinum doublet showed benefit in the ERCC1-negative and the ERCC1-positive population of patients.10 In 1995, the Non-Small-Cell Lung Cancer Collaborative Group (NSCLCCG) published a meta-analysis showing a strong trend in favor of adjuvant cisplatin-based chemotherapy (hazard ratio[HR], 0.87; 95% confidence interval [CI], 0.74–1.02; P = 0.08), with an absolute improvement in the 5-year survival rate of 5%. Although the benefit was not statistically significant, this observation prompted additional clinical trials to address the appropriate role of adjuvant chemotherapy in patients with resected NSCLC (Table 2).11 The standard of care for stage II– IIIA NSCLC patients is adjuvant cisplatin-based doublet chemotherapy after appropriate surgical resection to improve OS. The benefit for patients with stage IB NSCLC is less apparent, likely because of the heterogeneity of this population. The latest revisions to the TNM staging criteria should assist in risk stratification.11 Our data confirm the benefits of ERCC1 selectivity in improving the outcome of patients with NSCLC. Among the patients who received platinum-based therapy, those who were negative for ERCC1 had significantly longer overall survival than those who were positive as shown in Table 1. The degree of ERCC1-positive expression also correlated well with the overall survival, but this could also be attributed to the fact that most of our patient population (80%) received platinum-based therapy as per the standard guidelines.
Table 2.
Recent randomized phase III trials of adjuvant chemotherapy in NSCLC.
| TRIAL | N | STAGE | CHEMOTHERAPY | PORT | HR | P-VALUE |
|---|---|---|---|---|---|---|
| Positive trials | ||||||
| IALT18 | 1,867 | I–III | Cisplatin and vinca alkaloid or etoposide | Optional (30.6%) | 0.86 | 5-yr median follow-up <0.03 |
| JBR.1019 | 482 | IB–II | Cisplatin and vinorelbine | No | 0.69 | 5-yr median follow-up 0.04 |
| ANITA20 | 840 | IB–IIIA | Cisplatin and vinorelbine | Optional (28%) | 0.80 | 0.017 |
| Negative trials | ||||||
| ECOG 359021 | 488 | II–IIIA | Cisplatin and etoposide | All pts | 0.93 | 0.56 |
| ALPI22 | 1,209 | I–IIIA | Mitomycin, vindesine, and cisplatin | Optional | 0.96 | 0.589 |
| BLT23 | 381 | I–IIIA | Cisplatin based | Optional | 1.02 | 0.90 |
| CALGB 963324 | 344 | IB | Carboplatin and paclitaxel | No | 0.83 | 0.12 |
| Phase III of RRM1 and ERCC125 | 275 | IV–IIIB | ERCC1 and RRM1 expression–Based Chemotherapy Versus Gemcitabine/Carboplatin | No | N/A | 0.66 |
Abbreviations: ALPI, Adjuvant Lung Project Italy; ANITA, Adjuvant Navelbine International Trialist Association; BLT, Big Lung Trial; CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IALT, International Adjuvant Lung Trial; LACE, Lung Adjuvant Cisplatin Evaluation; NSCLC, non-small-cell lung cancer; PORT, postoperative radiotherapy.
We were unable to test for ribonucleotide reductase messenger RNA (RRM1) expression, the marker of resistance to gemcitabine therapy.12,13 In spite of that, our patient population with advanced NSCLC showed better survival when treated with gemcitabine, whether first or second line, compared to other regimens (19 versus 13 months; P = 0.003). This contrasts with the group of patients receiving platinum therapy, in whom platinum therapy was only significantly associated with improved overall survival in ERCC1-negative patients. This is the first study, however, that shows that gemcitabine therapy would influence the overall survival of patients with advanced NSCLC if we do not have the tools to test for the specific RRM1 unlike ERCC1 and platinum therapy.
The VEGF (VEGF-A, -C, -D) and the VEGF receptors (VEGFR-1, -2, and -3) are important molecular markers in angiogenesis and lymphangiogenesis. A study by Donnem et al.14 elucidated the prognostic significance of these molecular markers in tumor cells, as well as in the tumor stroma of resected NSCLC tumors. In that study, high tumor cell expression of VEGF-A, VEGFR-1, VEGFR-2, and VEGFR-3 was a significant negative prognostic indicator of disease-specific survival (DSS).14 In tumor stroma, however, high expression of VEGF-A, VEGF-C, VEGF-D, VEGFR-1, and VEGFR-2 correlated with good prognosis. In multivariate analyses, high expression of VEGFR-3 in tumor cells was an independent negative prognostic factor for DSS, whereas in stromal cells, high VEGF-C had an independent positive association with survival.14 In our study, however, the tumor tissue was obtained at biopsy for advanced stage disease. The abundance of tumor tissue made the discrimination between the tumor stromal tissue and the tumor cells very challenging. Our data regarding VEGF and VEGF-R tumor expression were not statistically significant in relation to overall survival; this could be attributed not only to the limited patient population, but also to the limited volume of tumor tissue obtained during biopsy rather than surgical resection. This might lead to the conclusion that VEGF and VEGF-R expression data should be reviewed with caution especially given data on stromal or tumor tissue expression as well as available information on the amount of available tumor tissue examined prior to any conclusions for prognosis or selection of therapy.
In another study on 115 patients, serum levels of 14-3-3σ were associated with a significantly longer survival in the methylation-positive group (15.1 versus 9.8 months; P = 0.004).15 In our study, however, we tested 14-3-3σ in paraffin-embedded tumor tissue, and both the positive and negative groups had similar survival. This suggests that the expression of 14-3-3σ may be of more value for prognostic stratification of patients with NSCLC if measured in the serum rather than by IHC analysis of paraffin-embedded tissues.
Over-expression of pAKT and loss of PTEN expression in NSCLC have been previously correlated with poor differentiation, lymph node involvement, distant metastasis, late stages as well as worse overall survival.16 Unfortunately, our study did not confirm these findings. In fact, both negative and positive expressions of both receptors had almost equal survival.
NF-kB, also known as p65, is a key transcription factor thought to play a major role in carcinogenesis, and its expression was shown to be significantly higher in advanced TNM stages (III–IV) than in earlier stages (I–II).16 In that study, 394 formalin-fixed and paraffin-embedded specimens from surgically resected lung tumors were examined. The overall survival analysis in patients with NSCLC (n = 298) showed that NF-kB did not significantly influence the overall survival or disease-free survival.16 Our results, however, are only relevant to the advanced stages of NSCLC (stage III, IV). In our study, patients with positive expression of NF-kB had a median survival of 9.9 months compared to 13 months in those with negative expression. However, only 4 of 46 patients had positive expression, making this a limited sample to draw broader conclusions. A larger scale study of NF-kB expression is warranted, especially with the ongoing research in targeted therapy utilizing NF-kB inhibitors in patients with lung cancer.17 For the future implications of our study, we are currently investigating the role of pharmacogenomics in relation to the k-ras mutation and have presented an abstract at IASLC/AACR (Abstract) and the potential use of these markers if used in combination with molecular profiling.
Limitation of the Study
Our study is retrospective, limiting the measurement of some key statistics. As a one-center study, the generalization of the results is restricted. Most of the patients with advanced stage were only diagnosed with fine needle aspiration or small biopsies, and thus limited tissue was available for further studies.
Conclusion
Gemcitabine therapy, ERCC1 negativity and treatment with platinum therapy, ECOG PS 0 or 1, and female gender correlated with improved overall survival in patients with advanced NSCLC.
Footnotes
Author Contributions
Conceived and designed the experiments: NK, MS, AM. Analyzed the data: MR. Wrote the first draft of the manuscript: NK, AZ, MS, MA. Contributed to the writing of the manuscript: PP, SS, HB, NP, MA. Agree with manuscript results and conclusions: NK, MS, AZ, PP, SS. Jointly developed the structure and arguments for the paper: PP, SS, AZ, MR, AM. Made critical revisions and approved final version: NK, MS, HB, NP. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51(1):15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Non-small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330(3):153–8. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 4.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 5.Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8(7):2286–91. [PubMed] [Google Scholar]
- 6.Papadaki C, Sfakianaki M, Ioannidis G, et al. ERCC1 and BRAC1 mRNA expression levels in the primary tumor could predict the effectiveness of the second-line cisplatin-based chemotherapy in pretreated patients with metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7(4):663–71. doi: 10.1097/JTO.0b013e318244bdd4. [DOI] [PubMed] [Google Scholar]
- 7.Roth JA, Carlson JJ. Prognostic role of ERCC1 in advanced non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer. 2011;12(6):393–401. doi: 10.1016/j.cllc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356(8):800–8. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 9.Seto T, Higashiyama M, Funai H, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53(1):91–6. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368(12):1101–10. doi: 10.1056/NEJMoa1214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangha R, Price J, Butts CA. Adjuvant therapy in non-small cell lung cancer: current and future directions. Oncologist. 2010;15(8):862–72. doi: 10.1634/theoncologist.2009-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell R, Cobo M, Isla D, Camps C, Massuti B. Pharmacogenomics and gemcitabine. Ann Oncol. 2006;17(suppl 5):v13–6. doi: 10.1093/annonc/mdj942. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Danenberg KD, Alberola V, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2004;10(4):1318–25. doi: 10.1158/1078-0432.ccr-03-0156. [DOI] [PubMed] [Google Scholar]
- 14.Donnem T, Al-Saad S, Al-Shibli K, et al. Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res. 2007;13(22 pt 1):6649–57. doi: 10.1158/1078-0432.CCR-07-0414. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez JL, Rosell R, Taron M, et al. 14-3-3 sigma methylation in pretreatment serum circulating DNA of cisplatin-plus-gemcitabine-treated advanced non-small-cell lung cancer patients predicts survival: The Spanish Lung Cancer Group. J Clin Oncol. 2005;23(36):9105–12. doi: 10.1200/JCO.2005.02.2905. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Liu D, Shishodia S, et al. Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107(11):2637–46. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 17.Suthar SK, Sharma N, Lee HB, Nongalleima K, Sharma M. Novel dual inhibitors of nuclear factor-kappa B (NF-kappaB) and cyclooxygenase- 2 (COX-2): synthesis, in vitro anticancer activity and stability studies of lantadene-non steroidal anti-inflammatory drug (NSAID) conjugates. Curr Top Med Chem. 2014;14(8):991–1004. doi: 10.2174/1568026614666140324120503. [DOI] [PubMed] [Google Scholar]
- 18.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 19.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28(1):29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 21.Keller SM, Adak S, Wagner H, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med. 2000;343(17):1217–22. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95(19):1453–61. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 23.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26(1):173–82. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bepler G, Williams C, Schell MJ, et al. Randomized international phase III trial of ERCC1 and RRM1 expression-based chemotherapy versus gemcitabine/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(19):2404–12. doi: 10.1200/JCO.2012.46.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]


