Figure 5.
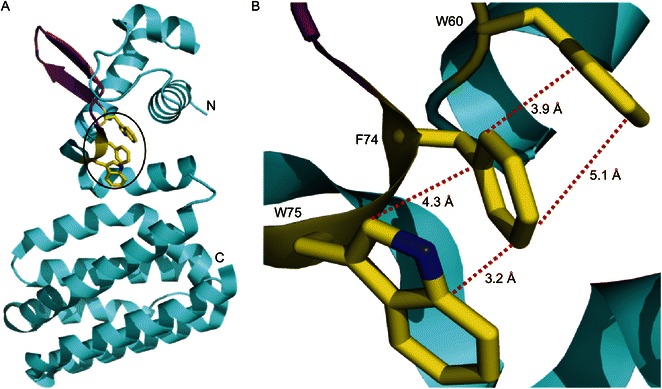
Structural analyses of FadR protein suggest that a hydrophobic interaction amongst W60, F74 and W75 might be important for its DNA binding activity. (A) Ribbon structure of E. coli FadR in monomer. N: N-terminus; C: C-terminus. α-Helix is blue, β-sheet is purple, and the three amino acids (W60, F74 and W75) that were proposed in this study is highlighted with a circle. (B) The enlarged view of the three critical residues (W60, F74 and W75). The hydrophobic bonds are expressed with dotted red lines, and the instance between two relevant atoms is labeled (angstrom). The photography was generated by Pmol software using the crystal structure’s PDB file of FadR protein (PDB: 1E2X)
