Figure 4.
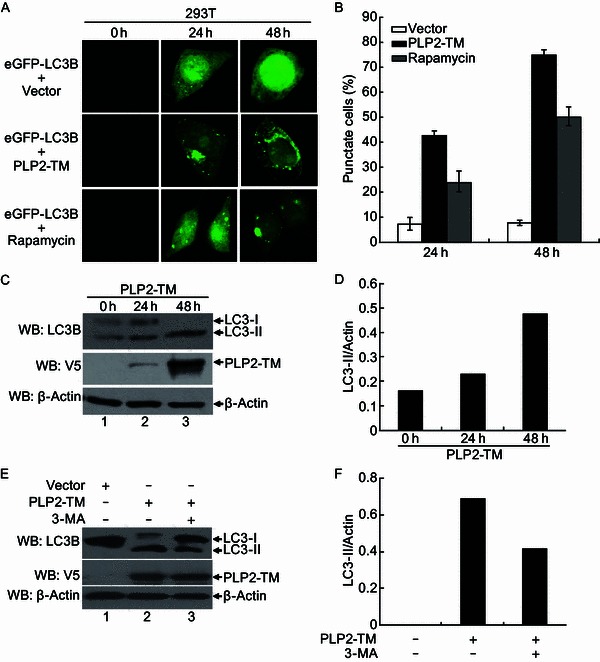
PLP2-TM induces autophagy formation in a time-dependent manner. (A) Immunofluorescence microscopy was used to detect the eGFP-LC3B-autophagosome in PLP2-TM transfected cells at different time point post-transfection. The plasmid of PLP2-TM-V5 was co-transfected with pcDNA3.1-eGFP-LC3B into HEK2923T cells. The cells were fixed at 0 h, 24 h and 48 h post-transfection, respectively and then analyzed for eGFP-LC3B positive autophagosome accumulation using a confocal microscope as described in Fig. 1B. (B) Quantification of cells displaying eGFP-LC3B puncta from one representative experiment that shown in Fig. 4A as described in Fig. 1B. (C and D) HEK293T was transfected with PLP2-TM-V5 or empty vector as a negative control. At 0 h, 24 h and 48 h post-transfection, the cells were then lysed for Western blotting analysis using a rabbit anti-LC3 antibody to detect the endogenous LC3 expression (top panel in Fig. 4C). The whole cell lysate (WCL) was blotted using anti-V5 antibodies to evaluate expression of PLP2-TM (middle panel in Fig. 4C), and β-Actin was detected in whole cell lysate (WCL) as a loading control (bottom panel in Fig. 4C). The band intensity was semi-quantitated by densitometric analysis using ImageJ software. (E and F) HEK293T cells were pretreated by 250 µmol/L 3-MA, an autophagy inhibitor, for 1.5 h and then transfected with PLP2-TM or empty vector for 48 h. The LC3, PLP2-TM and β-Actin were detected and semi-quantitated as described above
