Abstract
Increased plasma lactate levels can indicate the presence of metabolic disorders in HIV infected individuals.
Objective
To determine whether a portable analyzer is valid for measuring cerebrospinal fluid (CSF) and plasma lactate levels in HIV infected individuals.
Method
CSF and plasma were collected from 178 subjects. Samples tested by the Accutrend® portable analyzer were compared to those tested by a reference device (SYNCHRON LX® 20).
Results
The portable analyzer had in plasma sensitivity of 0.95 and specificity 0.87. For CSF the specificity was 0.95; the sensitivity 0.33; the negative predictive value was 95% and the positive predictive value 33%.
Conclusions
These findings support the validity of the portable analyzer in measuring lactate concentrations in CSF that fall within the normal range. The relatively poor positive predictive value indicates that a result above the reference range may represent a “false positive test”, and should be confirmed by the reference device before concluding abnormality.
Keywords: HIV, cerebrospinal fluid, clinical chemistry, lactate
The use of combination of antiretroviral (ARV) therapy (cART) has proven to be highly effective in slowing the progression to AIDS, reducing the incidence of opportunistic infections, and improving survival. However, several toxic effects of ARVs have been identified. The use of nucleoside reverse transcriptase inhibitors (NRTIs), one class of ARVs, can cause mitochondrial dysfunction which has been linked to hyperlactatemia and lactic acidosis, hepatic steatosis, lipoatrophy, peripheral neuropathy, HIV-associated neuromuscular weakness syndrome, pancreatitis, skeletal myopathies and cardiomyopathy1. NRTIs act as nucleoside analogues that stop HIV from replicating by becoming incorporated into HIV RNA strands and disallowing the attachment of additional nucleosides. NRTIs can also become incorporated into cell and mitochondrial DNA and inhibit replication. The inhibition of mitochondrial DNA polymerase-gamma leads to mitochondrial dysfunction, which causes cells to generate energy by anaerobic respiration, and results in increased levels of lactate2,3,4,5.
Mild to moderate hyperlactatemia (elevated plasma lactate) is seen in 8% to 21% of HIV-infected individuals taking NRTIs6. A more serious complication may occur in individuals with hepatic dysfunction due to drug related toxicities or chronic hepatitis due to reduced hepatic clearance of plasma lactate, requiring discontinuation of NRTIs. Several studies have suggested that routine monitoring of plasma lactate levels may be beneficial for identifying hyperlactatemia and thus NRTI related toxicity7. Increased levels of cerebrospinal fluid (CSF) lactate are associated with increased severity of symptoms of mitochondrial damage8, however, it has not yet been determined whether routine monitoring of CSF lactate, in addition to monitoring plasma lactate, would provide added benefits. Since CSF lactate and plasma lactate are produced independently, monitoring of plasma lactate alone may not be sufficient for determining whether mitochondria damage is specific to the central nervous system.
Many HIV care providers may not have easy access to commercial laboratories because of location or cost. Additionally, lactate measurements are sensitive to delays between sample collection and sample testing because of continuing metabolic activity after collection. Point-of-care testing can address these issues by providing quick, inexpensive and accurate results and can be performed using commercially available portable lactate analyzers. The reliability, validity and clinical utility of these measurements in blood have been well-validated for use in HIV uninfected individuals9,10,11,12 and has recently been evaluated in individuals with HIV with somewhat mixed results13,14. The use of portable analyzers for measuring CSF lactate levels has not been evaluated. The objective of this study was to validate the Accutrend® Lactate analyzer for measuring plasma and CSF lactate in a sample of HIV positive and negative individuals.
METHOD
Lactate was measured in 168 plasma samples and 47 CSF samples collected from 147 HIV positive and 31 HIV negative individuals prospectively enrolled in observational research studies at the HIV Neurobehavioral Research Center (HNRC) at the University of California, San Diego (UCSD). Research was approved by the local Institutional Review Board and informed consent was given by all study participants.
Plasma and CSF samples were collected by venipuncture and lumbar puncture respectively. For plasma venipuncture subjects sat for 10 to 20 minutes prior to the blood draw. A tourniquet was applied for less than one minute. Samples were collected into BD Vacutainer® (reference number 368521), which contain a combination of sodium fluorite (Na2 F) and sodium EDTA (Na2 EDTA). CSF samples were collected in sterilized glass tubes without anticoagulant. Samples were stored on ice immediately after collection. Samples were split and then tested using the Accutrend® Portable Lactate Analyzer and the SYNCHRON LX® 20 at the UCSD Medical Center. Portable analyzer measurements were completed on-site immediately, generally within 10 minutes of the sample being drawn. Reference device measurements were completed after delivery to the nearby, but off-site laboratory, within 1 hour of sample collection, transported on ice.
Portable analyzer: Accutrend® Lactate
The Accutrend® Lactate analyzer (formerly known as Accusport portable lactate analyzer; Roche, Boehringer Mannheim, Indianapolis, IN), uses enzymatic determination and reflectance photometry (wavelength 660 nm). The system reads the lactate levels in the plasma portion of whole blood. After sample processing, 20–25 µl of fluid is placed on the lactate test strip. A glass-fiber layer removes red blood cells before fluid continues to the final layer where a reaction takes place in the detector film resulting in a color change. Lactate is measured by reflectance photometry via a colorimetric lactate-oxidase mediator reaction. Results are provided within 60 seconds. The range of measurement for the analyzer is from 0.7 to 27 mmol/L.
Reference device: SYNCHRON LX® 20
The SYNCHRON LX® 20 (Beckman Coulter, Fullerton, CA) measures lactate in plasma and CSF. The assay converts lactate to pyruvate and hydrogen peroxide via lactate oxidase. The hydrogen peroxide reacts with dichlorobenzene-sulfonic acid and 4-aminoantipyrine in the presence of peroxidase to form a chromophore. The color change is measured by a wavelength of 520 nm. The assay requires 1 ml of fluid. The sensitivity range of the assay is 0.01–11.0 mmol/L. The assay is complete within 12–13 minutes.
Statistical analysis
The correlation between portable analyzer and reference device results was analyzed using Spearman’s Rho because lactate values in plasma and CSF were not normally distributed. The difference between the portable analyzer and reference device was evaluated by t-tests for matched pairs since the differences followed a normal distribution. Bias was evaluated by plotting the difference between the two measurements against the mean, as recommended by Altman and Bland15. Clinical agreement was defined as both measurements falling within or outside the reference interval of lactate for CSF and plasma. Although experts do not agree on the diagnostic threshold for lactate concentrations, the generally accepted reference range for CSF is <2.8 mmol/L and for plasma is <2.2 mmol/L16. The statistical program used for analysis was JMP version 7.0 (SAS Institute Inc.).
RESULTS
Subject characteristics are described in Table. Eighty-five percent (142/168) of plasma samples and 81% (38/47) of CSF samples came from HIV+ subjects. Lactate levels measured by the reference device ranged from 0.55–6.03 mmol/L for plasma and 1.07–6.53 mmol/L for CSF. The median plasma value was 3.32 mmol/L (inter-quartile range [IQR]=2.89–3.89). The median CSF value was 1.78 mmol/L (IQR=1.59–2.19).
Table.
Subject characteristics (n=178).
| Variable | Mean (SD) | Number (%) | Median (IQR) |
|---|---|---|---|
| Age, years | 44.38 (9.29) | – | – |
| Male | – | 142 (80%) | – |
| HIV positive | – | 147 (83%) | – |
| AIDS Diagnosis | – | 58 (39%)1 | – |
| CD4 Count | – | – | 474.5 (277.5–752.5) |
| On ARVs | – | 97 (66%) | – |
Reported for HIV+subjects only. SD: standard deviation; ARV: antiretroviral.
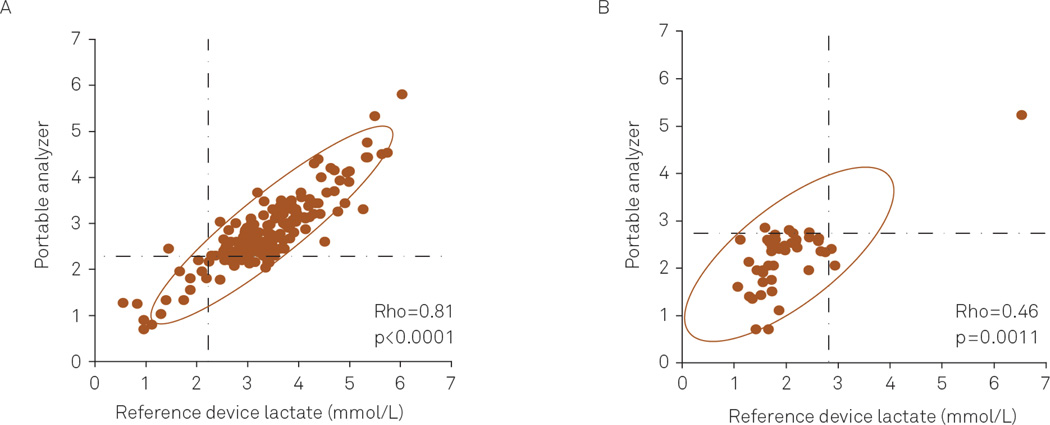
Lactate levels measured by the portable analyzer were correlated with measurements performed by the clinical laboratory (plasma: Spearman Rho=0.81, p<0.0001; CSF: Spearman Rho=0.46, p=0.0011) (Figure 1). For each pair of samples measured, portable lactate analyzer results were subtracted from the reference device results to determine whether a difference existed between the two measurements. For both fluids, the portable analyzer gave slightly, but significantly, different values (Plasma: mean difference=0.56 mmol/L, standard deviation=0.46 mmol/L, p<0.0001; CSF mean difference=−0.25 mmol/L, standard deviation=0.60 mmol/L, p=0.0051).
Figure 1.
Correlation of plasma (A) and cerebrospinal fluid (CSF) (B) lactate levels measured by the portable analyzer and reference device. The dashed horizontal and vertical lines indicate the cutoff for normal lactate levels in plasma and CSF.
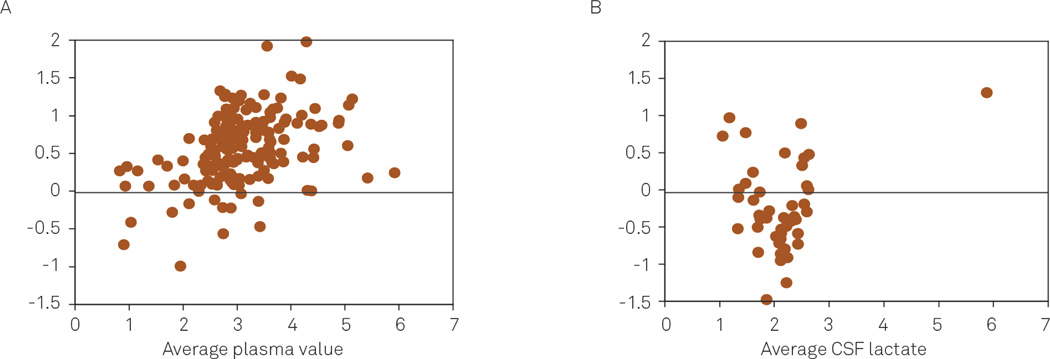
To determine whether the difference was related to the amount of lactate in plasma and CSF, the difference between measurements was plotted against the average of the two measurements (Figure 2). For plasma, the tendency of the reference values to exceed the portable analyzer results increased as the average of the paired measurements increased (Spearman Rho=0.40, p<0.0001). The difference between CSF measurements was not correlated with an increase in the average amount of CSF lactate (Spearman Rho 0.02, p<0.884).
Figure 2.
Altman-Bland plots comparing lactate levels measured in plasma (A) and cerebrospinal fluid (CSF) (B) by the portable analyzer and reference device.
Lactate levels from both devices were categorized according to whether they fell within or outside of the reference interval for lactate levels. Overall, agreement between the portable analyzer and reference device measurements occurred 91% of the time for CSF and 94% of the time for plasma. The sensitivity and specificity of the portable analyzer in correctly identifying abnormal lactate levels were 0.33 and 0.95 for CSF, and 0.95 and 0.87 for plasma. For pairs of measurements that did not agree, the median difference in measurements was −0.13 mmol/L for CSF and 0.80 mmol/L for plasma. For CSF, the negative predictive value was high (95%) and the positive predictive value was low (33%). For plasma, the negative and the positive predictive values were relatively high (98.6% and 61.9% respectively).
DISCUSSION
The results of this study generally support the use of the Accutrend® Lactate portable analyzer for point-of-care CSF and plasma lactate measurements. The accuracy of the portable analyzer was assessed using four methods: (1) evaluating the correlation between devices; (2) determining whether there was a difference between the two measurements; (3) evaluating whether the difference changes with the magnitude of the measurement; and (4) evaluating clinical agreement based on whether values fell within the reference ranges. Although some differences were seen between portable analyzer and reference device measurements for plasma, these differences were small (0.5 mmol/L on average) and did not diminish clinical validity (the portable analyzer had excellent sensitivity and specificity).
For CSF measurements, differences between the portable analyzer and reference device were also small and the portable analyzer showed excellent specificity for correctly identifying values in the normal range but did not have good sensitivity. This may be due to the fact, that in this subject population only 3 out of 47 CSF values were in the abnormal range. Even though pairs of CSF measurements did not fully agree in terms of whether or not the result fell within the reference range, the average difference between measurements in disagreement was small (−0.13 mmol/L). To maximize the usefulness of the portable analyzer for CSF users should consider the clinical significance of results that approach the limit of reference range in addition to results that exceed the limit of the reference range.
The small number of abnormal values for CSF limits the ability of the current findings to confidently assess sensitivity and positive predictive values, however, our results indicate that the portable analyzer can still accurately determine lactate concentration that fall within the normal range, which has clinical value.
Several other studies have shown that the Accutrend® Portable Analyzer provides accurate plasma and blood lactate measurements in athletics, critical care, obstetrics and other settings9,10,11,12,17,18. In these studies the portable analyzer produced results comparable to reference devices (such as the one in this study) and other portable analyzers19,20 (e.g. Lactate Pro). A study by Moore et al.13, evaluated the use of the portable analyzer for measuring blood lactate in an HIV infected population. Portable analyzer results were highly correlated with, but slightly higher than, the reference device. Despite the small difference in lactate levels, the portable analyzer measurements had strong clinical validity; they were highly predictive of mortality in patients with sepsis. Another study in HIV infected individuals found the portable lactate analyzer to have excellent sensitivity, but lower specificity, for identifying hyperlactatemia14. Despite slight variations in the prior findings (for example, in terms of sensitivity, specificity) taken together the research generally supports the validity of the portable analyzer for blood lactate. Consistent with prior research, our study confirms the validity of the portable analyzer for measuring blood lactate and extends previous findings by examining its use in CSF.
NRTIs have the potential for causing metabolic dysfunction but are still widely used, especially in resource poor settings. Detecting metabolic complications early can provide an opportunity for preventing symptom development and long-term complications. Early detection is accomplished by routine lactate measurements, which is facilitated by the availability and demonstrated accuracy of portable lactate analyzers.
Despite the importance of routinely monitoring lactate levels, the limitations of traditional measurements make it impractical for many settings. The portable analyzer has many advantages making it a better option for research and clinical settings that lack funds or access to a clinical laboratory. It is easy to use and can be operated by non-technicians with minimal training. The Accutrend® Portable Analyzer is inexpensive costing approximately $235 for the analyzer and as low as $1.14 per test strip compared to $91 per sample charged by the commercial laboratory. It reduces turn around time and quickens clinical decision-making. Because it is portable, samples can be tested immediately after collection, which minimizes error related to ongoing production of the lactate.
The purpose of this study was to assess the portable lactate analyzer as a screening tool by comparing it to a more reliable and accurate standard. The strong clinical agreement and correlation demonstrated for plasma confirms what other studies have shown about the validity of this portable analyzer. The portable analyzer applied to CSF had a relatively good negative predictive value (95%), indicating that a result within the reference range is likely to test normal also with the reference device. The relatively poor positive predictive value (33%) indicates that a result above the reference range may represent a “false positive test”, and should be confirmed by the reference device before concluding abnormality. Together, these findings support the validity of the portable analyzer in measuring lactate concentrations in CSF that fall within the normal range. While these data do not indicate that the portable analyzer can reliably measure abnormally high lactate levels in CSF, the accurate identification of normal values enables exclusion of abnormal values, which may be valuable in resource limited settings when other methods are not readily available. Additional studies that focus on including a wider range of CSF lactate levels should be conducted to further validate the instrument.
Acknowledgments
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S., Christi Kao, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Support: The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
Footnotes
Conflict of interest: There is no conflict of interest to declare.
References
- 1.Mallon PW, Unemori P, Sedwell R, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–1696. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 2.Cherry CL, Lala L, Wesselingh SL. Mitochondrial toxicity of nucleoside analogues: mechanism, monitoring and management. Sex Health. 2005;2:1–11. doi: 10.1071/sh04016. [DOI] [PubMed] [Google Scholar]
- 3.Dagan T, Sable C, Bray J, Gerschenson M. Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: what is the evidence? Mitochondrion. 2002;1:397–412. doi: 10.1016/s1567-7249(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 4.Lewis W, Copeland WC, Day BJ. Mitochondrial dna depletion, oxidative stress, and mutation: mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab Invest. 2001;81:777–790. doi: 10.1038/labinvest.3780288. [DOI] [PubMed] [Google Scholar]
- 5.Moyle G. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin Ther. 2000;22:911–936. doi: 10.1016/S0149-2918(00)80064-8. [DOI] [PubMed] [Google Scholar]
- 6.Imhof A, Ledergerber B, Gunthard HF, Haupts S, Weber R. Risk factors for and outcome of hyperlactatemia in HIV-infected persons: is there a need for routine lactate monitoring? Clin Infect Dis. 2005;41:721–728. doi: 10.1086/432471. [DOI] [PubMed] [Google Scholar]
- 7.Montaner JS, Cote HC, Harris M, et al. Mitochondrial toxicity in the era of HAART: evaluating venous lactate and peripheral blood mitochondrial DNA in HIV-infected patients taking antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;34(Suppl):S85–S90. doi: 10.1097/00126334-200309011-00013. [DOI] [PubMed] [Google Scholar]
- 8.Vittecoq D, Jardel C, Barthelemy C, et al. Mitochondrial damage associated with long-term antiretroviral treatment: associated alteration or causal disorder? J Acquir Immune Defic Syndr. 2002;31:299–308. doi: 10.1097/00126334-200211010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Pines JM, Drumheller BC, Gaieski DF. Point-of-care testing at triage decreases time to lactate level in septic patients. J Emerg Med. 2010;38:578–581. doi: 10.1016/j.jemermed.2007.11.099. [DOI] [PubMed] [Google Scholar]
- 10.Mc Naughton LR, Thompson D, Philips G, Backx K, Crickmore L. A comparison of the lactate Pro, Accusport, Analox GM7 and Kodak Ektachem lactate analysers in normal, hot and humid conditions. Int J Sports Med. 2002;23:130–135. doi: 10.1055/s-2002-20133. [DOI] [PubMed] [Google Scholar]
- 11.Medbo JI, Mamen A, Holt Olsen O, Evertsen F. Examination of four different instruments for measuring blood lactate concentration. Scand J Clin Lab Invest. 2000;60:367–380. doi: 10.1080/003655100750019279. [DOI] [PubMed] [Google Scholar]
- 12.Yam J, Chua S, Razvi K, Arulkumaran S. Evaluation of a new portable system for cord lactate determination. Gynecol Obstet Invest. 1998;45:29–31. doi: 10.1159/000009919. [DOI] [PubMed] [Google Scholar]
- 13.Moore CC, Jacob ST, Pinkerton R, et al. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis. 2008;46:215–222. doi: 10.1086/524665. [DOI] [PubMed] [Google Scholar]
- 14.Perez EH, Dawood H, Chetty U, Esterhuizen TM, Bizaare M. Validation of the accutrend lactate meter for hyperlactatemia screening during antiretroviral therapy in a resource-poor setting. Int J Infect Dis. 2008;12:553–536. doi: 10.1016/j.ijid.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Bland JM. Measurment in medicine: the analysis of method comparison studies. The Statistician. 1983;32:307–317. [Google Scholar]
- 16.Tietz NW. Clinical Guide to Laboratory Tests. 3rd ed. Philadelphia, PA: WB Saunders Company; 2006. p. 1995. [Google Scholar]
- 17.Boldt J, Kumle B, Suttner S, Haisch G. Point-of-care (POC) testing of lactate in the intensive care patient. Accuracy, reliability, and costs of different measurement systems. Acta Anaesthesiol Scand. 2001;45:194–199. doi: 10.1034/j.1399-6576.2001.450210.x. [DOI] [PubMed] [Google Scholar]
- 18.Terblanche E, Cloete WA, du Plessis PA, Sadie JN, Strauss A, Unger M. The metabolic transition speed between backward walking and running. Eur J Appl Physiol. 2003;90:520–525. doi: 10.1007/s00421-003-0890-7. [DOI] [PubMed] [Google Scholar]
- 19.Buckley JD, Bourdon PC, Woolford SM. Effect of measuring blood lactate concentrations using different automated lactate analysers on blood lactate transition thresholds. J Sci Med Sport. 2003;6:408–421. doi: 10.1016/s1440-2440(03)80267-0. [DOI] [PubMed] [Google Scholar]
- 20.Dascombe BJ, Reaburn PR, Sirotic AC, Coutts AJ. The reliability of the i-STAT clinical portable analyser. J Sci Med Sport. 2007;10:135–140. doi: 10.1016/j.jsams.2006.05.023. [DOI] [PubMed] [Google Scholar]