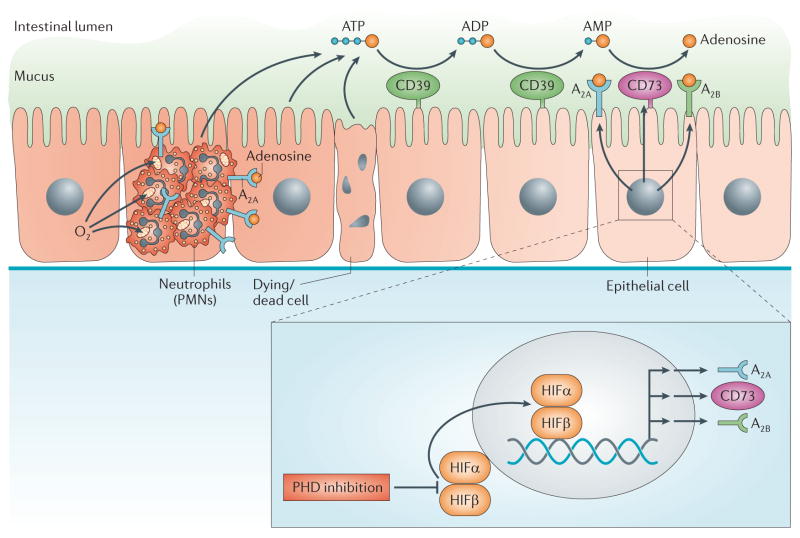
Figure 2. PHD inhibitor treatment of inflammatory bowel disease.
Inflammatory bowel disease is a result of intestinal inflammation, in which profound changes in metabolic supply and demand lead to an imbalance in oxygen supply. This imbalance causes severe hypoxia of the inflamed mucosa. Mucosal hypoxia during intestinal inflammation can be visualized using nitroimidazole compounds that stain hypoxic tissues33,34. Infiltrating polymorphonuclear neutrophils (PMNs) contribute to tissue hypoxia of the inflamed intestinal mucosa by localized oxygen depletion and concomitant stabilization of hypoxia-inducible factor (HIF), thereby contributing to the resolution of inflammation51. Inflammatory hypoxia causes the activation of hypoxia-elicited gene programmes. Coordinated gene programmes result in the increased production and signalling of extracellular adenosine. This gene programme is under the control of SP1-dependent induction of CD39, HIF-dependent induction of CD73 and the adenosine A2A and A2B receptors. These transcriptional changes lead to an increased turnover rate of the extracellular nucleotides ATP and ADP to AMP (via CD39) and subsequently via CD73 to adenosine. This pathway provides robust protection during intestinal inflammation. Importantly, orally available inhibitors of prolyl hydroxylases (PHDs) are available for the treatment of patients and are effective in increasing erythropoietin responses. Such compounds can promote intestinal protection from inflammation via enhancing extracellular adenosine production and signalling through adenosine receptors.