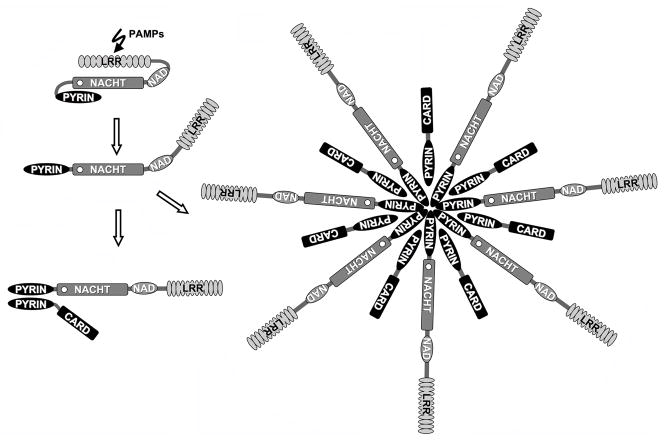
Figure 3. A proposed model for the activation of PAN proteins.
PAN proteins probably exist as inactive proteins. This status is most likely mediated by the LRR domain, which presumably blocks access to the NACHT domain. This conformation might resemble APAF-1 and R proteins. The effector domain (PYRIN domain) is tightly packed against the NACHT domain, and is therefore inaccessible for binding partners. Ligand binding could release inhibition by the LRR domain, and results in unfolding of these domains. A requirement for NTP binding and hydrolysis is anticipated, but has not been demonstrated. Once unfolded, the PYRIN domain is able to recruit adapter proteins, such as ASC. Activation of downstream effectors is initiated by the recruitment of ASC-linked effectors, as described for the R-gene product Rx of Solanaceae [47, 170]. However, an alternative mechanism could be the oligomerization of PAN-family proteins, which may activate effectors by the enhanced dimerization mechanism, as described for APAF-1 [169].