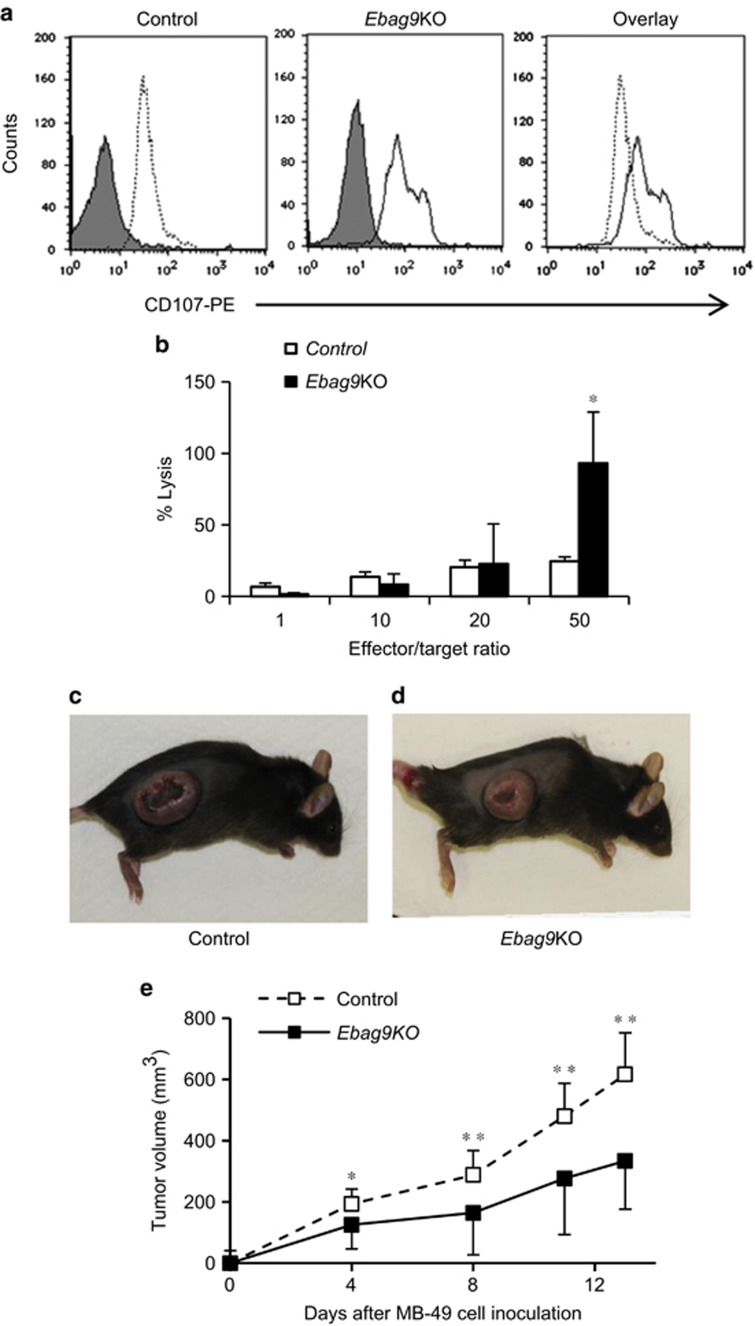
Figure 6.
Promotion of degranulation and cytotoxic activity of CD8+ T cells by EBAG9 knockout. (a) Flow cytometry-based degranulation assay of CD8+ T cells from tumors in control (left) and Ebag9KO (middle) mice using phycoerythrin (PE)-conjugated anti-CD107 antibody. Overlap (right) of anti-CD107-PE fluorescence in Ebag9KO and control CD8+ T cells illustrates the significant increase in degranulation due to Ebag9 deletion. The peaks with grey shading represent cells stained with IgG2a–PE as a negative control. (b) Cytotoxicity assay of CD8+ T cells from tumors in Ebag9KO and control mice. MB-49 cells (target) were incubated with CD8+ T cells (effector) prepared from tumors generated in Ebag9KO and control mice at various effector/target ratios. The cytotoxic activity of CD8+ T cells was monitored as the amount of lactate dehydrogenase in culture medium using the CytoTox 96 Non-Radioactive Cytotoxicity Assay. (c and d) CD8+ T cells isolated from tumors generated in Ebag9KO mice exhibits antitumor activity. C57BL/6 mice were intravenously injected the CD8+ T cells (2 × 106 cells per mouse) isolated from tumors generated in control (c) or Ebag9KO mice (d) and 2 days later, MB-49 cells were subcutaneously implanted into the recipient mice (5 × 105 cells per mouse). Tumor volumes were calculated once or twice a week. (e) Adaptive transfer of CD8+ T cells from Ebag9KO mice significantly suppressed tumor growth by MB-49 cells implanted in C57BL/6 mice compared with those from control mice. The data shown are mean values±s.d. *P<0.05, **P<0.01 by Student's t-test.