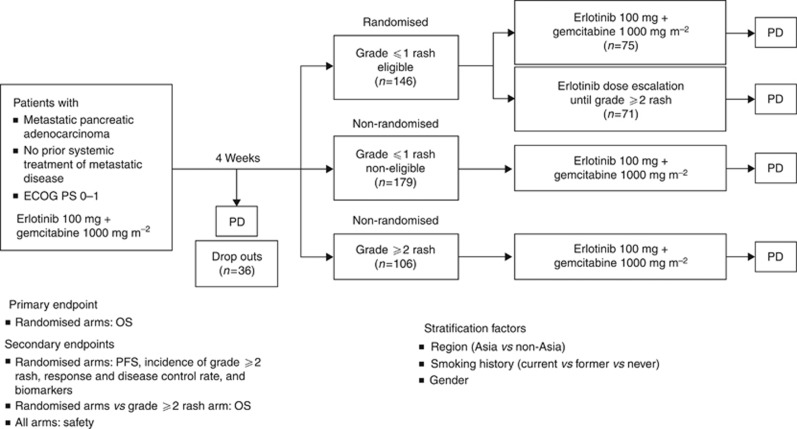
Figure 1.
Study design of the phase II RACHEL (BO21128) study. The 4-week run-in period was based on a median onset for rash development in patients treated with erlotinib plus gemcitabine for 10 days (with 90% of patients experiencing rash within 44 days) seen in the PA.3 study (Wacker et al, 2007). Erlotinib dose escalation—starting at 150 mg per day increasing at 50 mg every 2 weeks until a maximum of 250 mg per day or grade ⩾2 rash developed (patients were held at the lowest dose that induced grade ⩾2 rash). A 50-mg increment allowed monitoring of rash development/other toxicities and ensured patients were not exposed to unnecessarily high doses of erlotinib. Abbreviations: ECOG PS=Eastern Cooperative Oncology Group performance status; OS=overall survival; PD=disease progression; PFS=progression-free survival.