Figure 1.
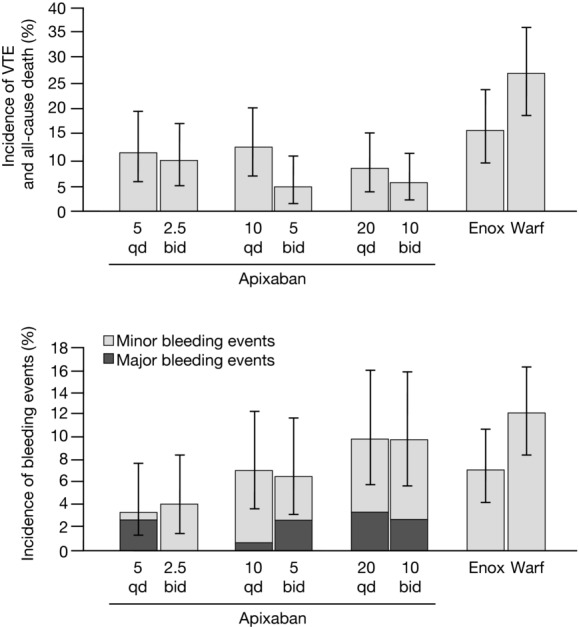
Efficacy and bleeding outcomes in the phase II dose-ranging trial of apixaban for prevention of VTE following elective knee replacement surgery (APROPOS trial). Three total daily dose levels of apixaban were tested (5, 10, 20 mg), administered either qd (5 mg qd, 10 mg qd, 20 mg qd) or in divided doses bid (2.5 mg bid, 5 mg bid, 10 mg bid). Reflecting clinical practice patterns in the United States, two comparators were tested: enoxaparin 30 mg bid (subcutaneous administration) and warfarin (target INR range of 2–3). The phase III dose studied in trials for prevention of VTE was 2.5 mg bid, and the phase III dose studied in trials for stroke prevention in AF was 5 mg bid. Modified, with permission, from Lassen et al.15
