Abstract
Background
Stimulus deprivation amblyopia (SDA) develops due to an obstruction to the passage of light secondary to a condition such as cataract. The obstruction prevents formation of a clear image on the retina. SDA can be resistant to treatment, leading to poor visual prognosis. SDA probably constitutes less than 3% of all amblyopia cases, although precise estimates of prevalence are unknown. In developed countries, most patients present under the age of one year; in less developed parts of the world patients are likely to be older at the time of presentation. The mainstay of treatment is removal of the cataract and then occlusion of the better-seeing eye, but regimens vary, can be difficult to execute, and traditionally are believed to lead to disappointing results.
Objectives
Our objective was to evaluate the effectiveness of occlusion therapy for SDA in an attempt to establish realistic treatment outcomes. Where data were available, we also planned to examine evidence of any dose response effect and to assess the effect of the duration, severity, and causative factor on the size and direction of the treatment effect.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 9), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2013), EMBASE (January 1980 to October 2013), the Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to October 2013), PubMed (January 1946 to October 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 October 2013.
Selection criteria
We planned to include randomized and quasi-randomized controlled trials of participants with unilateral SDA with visual acuity worse than 0.2 LogMAR or equivalent. We did not specify any restrictions for inclusion based upon age, gender, ethnicity, co-morbidities, medication use, or the number of participants.
Data collection and analysis
Two review authors independently assessed study abstracts identified by the electronic searches.
Main results
We did not identify any trials that met the inclusion criteria specified in the protocol for this review.
Authors’ conclusions
We found no evidence on the effectiveness of any treatment for SDA. Future randomized controlled trials are needed in order to evaluate the safety and effectiveness of occlusion, duration of treatment, level of vision that can be realistically achieved, effects of age at onset and magnitude of visual defect, optimum occlusion regimen, and factors associated with satisfactory and unsatisfactory outcomes with the use of various interventions for SDA.
Background
Description of the condition
The term ‘amblyopia’ refers to bluntness of vision and is derived from the Greek words “amblys” (meaning blunt) and “ops” (meaning eye). Clinically, amblyopia denotes a reduction in vision in the absence of any retinal anomaly or any disorder of the afferent visual pathways (Duke-Elder 1973). Amblyopia typically affects only one eye but it sometimes affects both eyes. Amblyopia is usually classified according to its cause:
strabismic, caused by squint (eye misalignment);
anisometropic, caused by unequal refractive (focusing) error;
meridional, caused by astigmatism (irregular corneal curvature);
ammetropic, caused by high refractive error in both eyes;
stimulus deprivation, caused by an obstruction in the visual pathway.
Mixed amblyopia, which is a result of more than one cause, is typically due to a combination of strabismic and anisometropic amblyopia. This review is focused on interventions for stimulus deprivation amblyopia (SDA), also called amblyopia ex anopsia. Other Cochrane reviews have evaluated interventions for strabismic and anisometropic (refractive) amblyopia (Taylor 2011; Taylor 2012).
Pathophysiology
Visual experiences in early life determine the organization of the portion of the adult brain that processes visual stimuli (Wiesel 1963). The time within which abnormal visual input can lead to a disruption of the normal pattern of development is called the ‘critical period’ (Hockfield 1998). There are several critical periods, each associated with different visual functions (Harwerth 1990), which probably reflect development of different parts of the brain. These critical periods can be considered as a continuum from extreme sensitivity to almost no sensitivity to external stimuli. Amblyopia begins to develop in these critical periods at young ages when the brain and the visual system are immature and connections between neurons are still being formed and stabilized. During the critical period, most amblyopia is reversible, usually until the child is 10 years old (AAO 2002). The critical period varies considerably among children and depends on the type of amblyopia.
Etiology
Patients with SDA lose vision from disuse or lack of formation of clear retinal images, most commonly as a result of one of the following:
unoperated infantile cataract (opacity of the lens);
ptosis (droopy eyelids) (Dray 2002; Gusek-Schneider 2000);
hemangioma (blood-rich swelling on the lid) (Schulz 1982);
obstruction in the vitreous (clear gel that fills the eye), for example due to bleeding (Ferrone 1994);
aphakia (absence of the natural lens);
occlusion prescribed to treat amblyopia of the other eye (Awaya 1973; Von Noorden 1973; Von Noorden 1981).
Patients with SDA may have either otherwise healthy eyes or other co-existing conditions, such as microphthalmos (small eye), coloboma (incomplete formation of the eye), optic nerve hypoplasia (under-developed optic nerve), or retinal abnormalities. Coexisting conditions often limit the visual prognosis. It is difficult to discern whether the visual loss is due to SDA or due to other co-existing conditions in the eye.
The most commonly reported cause of SDA is congenital or infantile cataract in one eye. Stimulus deprivation secondary to the cataract continues until the cataract is removed and optical correction is provided. Even after optical correction, the affected eye may continue to be anisometropic and anisekonic (forming unequal image sizes) (Enoch 1983). The early insult to the visual system is believed to make this type of amblyopia particularly severe and resistant to treatment. The visual prognosis has been reported to be poor (Kanski 1994; Taylor 1997).
Epidemiology
The prevalence of amblyopia in the general population ranges from 1% to 5% (Brown 2000; Hillis 1983). In European children, the prevalence ranges from 1% to 2.5% (Kvarnstrom 2001; Newman 2000). Amblyopia accounts for 29% of unilateral blindness in Copenhagen (Buch 2001) and as much as 8.3% of bilateral blindness in India following childhood cataract surgery (Dandona 2003). SDA accounts for less than 3% of amblyopic patients (Hillis 1983).
Presentation
Routine health checks of babies and toddlers are carried out by a variety of health care personnel (for example, pediatricians, nurses) and provide an opportunity for detection of the causative factors (for example, ptosis, cataract) associated with SDA. SDA itself is not likely to be noticed, but parents may detect the signs associated with causes of SDA such as leukocoria (white pupils) in children with congenital cataracts or droopy eyelid (ptosis). Children may also present with squint (misalignment) as a result of poor vision in one eye. In the developed world, most patients with SDA present for treatment while they are under the age of one (Mein 1991).
Diagnosis
There are four main steps in the diagnosis of SDA.
Visual acuity testing: testing young children largely relies on objective observations that are limited by cognition and concentration. Qualitative methods (e.g., assessing fixation preference) may be used, however quantitative tests (e.g., preferential looking) are more precise. Preferential looking tests rely on the observation that infants prefer to look at patterned rather than plain surfaces (Fanz 1958). Children look at a striped panel when they can discern it. A Snellen equivalent can then be computed using the degree of visual angle subtended by the stripes in the panel. In older children, testing methods are more objective and rely on the child's identification of pictoral or letter optotypes in Snellen, decimal or logMAR notation.
External and internal eye examination to identify any coexisting conditions: the examination must focus on identifying lesions such as optic nerve hypoplasia that could lead to inappropriate or unsuccessful treatment of the condition.
Cycloplegic refraction and corrective prescription when indicated: amblyopia can be diagnosed only after correcting any significant refractive error.
Rechecking visual acuity with any prescribed refractive correction in place: some improvement in visual acuity can be expected with refractive correction alone. There should be a period of adjustment to spectacles before retesting. Traditionally this adjustment period has been four to six weeks, but studies on refractive and strabismic amblyopia show this period may be as long as 24 weeks (Moseley 2002).
Definitions of amblyopia vary largely due to the fact that there is little evidence as to what constitutes normal vision at different ages based on successful performances on many commonly used tests. Amblyopia may be defined by comparing both eyes (inter-ocular difference) or by looking at monocular visual acuity alone. We have elected to define amblyopia as vision worse than 6/9 on a Snellen-based test, 0.2 LogMAR or its equivalent in one eye. Assuming that the fellow eye has normal vision, that is 6/6 or logMAR 0.0, this definition means that the difference in vision between the two eyes is greater than 0.2 log MAR.
Description of the intervention
Visual loss due to SDA can be difficult to quantify due to the limitations of the visual acuity tests available for young children but is believed to be severe in most cases. The aim of treatment is to maximize visual recovery without adversely affecting acuity in the better-seeing eye. The rationale for treatment is two-fold, to provide a good second eye should the better-seeing eye ever be visually compromised; and to maximize stereopsis (binocular cooperation between the eyes). Untreated or unsuccessfully treated amblyopia may affect adult life. For individuals with amblyopia, the lifetime risk of serious visual impairment due to loss or damage of the better-seeing eye is estimated to be between 1.2% and 3.3% (Rahi 2002). In addition, there are implications for employment prospects and, therefore, income. The number of jobs barred to individuals with reduced vision increases with the severity of the deficit (Adams 1999). Futhermore, stereopsis is required to participate in many sports and for some jobs.
Stages of treatment
The first stage is to correct any factor degrading the quality of the visual image (e.g., infantile cataract extraction, ptosis repair). In cases of early unilateral deprivation, correction must be undertaken in the first eight to 12 weeks of life (Birch 1986; Birch 1988; Gregg 1992; Kanski 1994; McCulloch 1994; Taylor 1997).
The second stage is to provide necessary refractive correction to maximize the quality of visual stimulation received by the child's amblyopic eye. Intraocular implants, contact lenses or both may be used after cataract surgery.
The third stage is occlusion therapy. Occlusion of the unaffected eye forces the use of the amblyopic eye to stimulate the formation of functional connections in the brain (Boothe 2000).
How the intervention might work
Occlusion regimen
Protocols and practices for occlusion therapy vary considerably. Typically, occlusion therapy ranges in duration from an hour to more than six hours (full-time). Factors affecting the prescribed amount of occlusion include the level of visual deficit, the age of the child and the likely waiting time to the next appointment. Follow-up is recommended at intervals of one week per year of age during periods of aggressive patching (Simon 1987). Occlusion may be stopped when visual acuity becomes equal in the two eyes or if no progress has been made after three months of good compliance with occlusion (Pratt-Johnson 2001). Children may require monitoring until they reach the age of visual maturity (approximately seven years of age) to ensure that amblyopia does not recur. Some periods of maintenance occlusion may be required during that time (Mein 1991).
The following have been used as additions to occlusion therapy, but are not currently popular clinically.
CAM visual stimulator: uses rotating high-contrast square wave gratings to stimulate the amblyopic eye.
Pleoptics: employs after-images to encourage foveal fixation and normal projection in the amblyopic eye.
Types of occlusion
Atropine penalization and optical penalization (patching or use of lenses to reduce the acuity) are forms of occlusion that encourage use of the amblyopic eye by diminishing visual form. These treatments for amblyopia were evaluated in another Cochrane review, which showed that atropine penalization is as effective as conventional occlusion (Li 2009). Total occlusion is not without disadvantages in terms of discomfort, but it is relatively easy to control the dosage of treatment and is without the more complex side effects of alternatives such as occlusive contact lenses.
Measuring outcomes
In order to quantify amblyopia, visual acuity must be measured. Qualitative methods for assessing vision in preverbal children are based on the observation of their fixation patterns. These methods are often unreliable and require highly trained examiners (Wright 1986; Zipf 1976). Visual acuity assessed using an age-appropriate test (Fulton 1978; Sebris 1987) is the most commonly used outcome to evaluate treatment for amblyopia. Tests vary in the use of optotypes (pictures, letters, or symbols), presented with or without crowding. Crowded visual acuity tests are harder to perform but are more sensitive to amblyopia than uncrowded tests.
Developmental changes in young children complicate the evaluation of actual change in visual acuity before and after treatment. Alternative methods of measuring change have been suggested (Schmidt 1994; Stewart 2003), but we aimed to compare post-treatment visual acuity levels (defining restoration of normal visual acuity as better than or equal to 6/9 on Snellen, or 0.2 LogMAR or its equivalent).
Factors affecting outcome
Compliance with therapy is critical for successful treatment but often can be difficult to achieve. Young children can become distressed by being restricted to reduced visual acuity and from the discomfort of wearing an adhesive patch. It has been suggested that, if possible, compliance should be monitored to measure its affect on the response to treatment. Devices to objectively measure compliance have been developed (Awan 2005; Stewart 2005) but are not in common use; typically clinicians depend on parental reports. Other factors believed to affect treatment success are the duration of visual deprivation, the age at onset and timing of initiation of therapy (Maurer 1989); early onset of amblyopia, long duration of the condition and late initiation of therapy are associated with worse visual prognosis.
Harm from occlusion therapy
Potential adverse effects from occlusion therapy include inducing amblyopia in the occluded eye, skin allergies, infections or corneal abrasions from contact lens wear, diplopia (double vision) and psychological effects on the parents and children (for example, distress).
Why it is important to do this review
The reported success of treatment for SDA varies. Studies have reported good levels of vision following early treatment (Gregg 1992; McCulloch 1994) but there is a lack of standardization and poor agreement among experts as to the optimum amount of occlusion needed to achieve a good visual outcome. Commencing occlusion therapy in infants with very poor vision can be harrowing for the parents and stressful for the child. Realistic treatment goals are often poorly defined. It is thus necessary to establish the most effective occlusion regimen(s) for stimulus deprivation amblyopia and to define the degree of improvement that can reasonably be expected from this treatment.
Obectives
Our objective was to evaluate the effectiveness of occlusion therapy for SDA in an attempt to establish realistic treatment outcomes. Where data were available, we also planned to examine evidence of any dose response effect and to assess the effect of the duration, severity and causative factor on the size and direction of the treatment effect.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized and quasi-randomized trials in this review, with no restriction on the number of participants in the trials.
Types of participants
We planned to include trials that recruited participants with the following characteristics.
Unilateral SDA, defined as best-corrected visual acuity in the affected eye worse than 6/9 Snellen, or its equivalent, after treatment for the causative factor had been undertaken and ensuing that refractive error had been corrected. We also planned to report other co-existing amblyogenic factors.
Unrestricted age, gender, ethnicity, co-morbidity, and medication use.
Types of interventions
We planned to include trials evaluating the following interventions:
total occlusion by adhesive patch;
total occlusion by occlusive contact lens;
pleoptic treatment;
partial occlusion (i.e., Bangerter filters);
CAM visual stimulation.
We planned to examine the following comparisons:
total occlusion versus no occlusion;
any method of total occlusion compared to another;
total occlusion plus pleoptic treatment versus total occlusion alone;
total occlusion plus CAM visual stimulator versus total occlusion alone;
full-time occlusion (more than six hours/day) versus part-time occlusion (less than six hours/day);
different durations of partial occlusion, for example two hours/day versus six hours/day;
any partial occlusion compared to another;
any total occlusion compared to any partial occlusion;
any partial occlusion compared to no occlusion.
Types of outcome measures
Primary outcomes
The primary outcome for this review was best-corrected visual acuity (BCVA) of the amblyopic eye, assessed by an age-appropriate test 12 months after cessation of occlusion therapy. Although the two are not directly equivalent, we planned to convert Snellen data into the logMAR equivalent for ease of interpretation and analysis.
We planned to dichotomize the outcomes as follows.
Normal = better than or equal to 0.2 logMAR, 6/9 Snellen or its equivalent.
Residual deficit = worse than 0.2 logMAR units.
Where possible, we planned to compare the proportions of children with the outcomes specified versus without.
Secondary outcomes
The secondary outcomes for this review were:
visual acuity in the amblyopic eye at seven years of age or older;
the proportion of the amblyopia deficit corrected (Stewart 2003);
any measure of stereoacuity (three-dimensional (3-D) vision).
Cost data
We planned to summarize in the following categories the comparative costs of the treatment methods described in the included trials.
Adverse effects
We planned to summarize adverse effects related to treatment that were reported in the included trials.
Severe: occlusion amblyopia, contact lens-related problems (e.g., infection, corneal abrasions), adverse psychological effects (e.g., distress), treatment cessation due to poor compliance or failure to attend follow-up visits, or diplopia (double vision).
Minor: allergy to patches.
Quality of life measures
We planned to summarize any data on quality of life measures of both the parents and child, as described in the reports of the included trials.
Follow-up
We planned to include in our analyses data from trials with a minimum post-treatment follow-up of six months. For trials that did not meet this criterion, we planned to include the trials in our qualitative synthesis but exclude them from any meta-analyses.
Search methods for identification of studies
Electronic searches
In 2013, we revised the searches of electronic databases from the 2007 update. We searched PubMed which had not originally been searched. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) 2013, Issue 9, part of The Cochrane Library. www.thecochranelibrary.com (accessed 28 October 2013), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily Update, Ovid OLDMEDLINE (January 1946 to October 2013), EMBASE (January 1980 to October 2013), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2013), PubMed (January 1946 to October 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrial.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 October 2013.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), PubMed (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and ICTRP (Appendix 8).
Searching other resources
We did not conduct any manual searches for this review. In future updates of this review, we will search the Web of Science® for any articles that cited reports of trials included in this review. In addition, we also will handsearch references cited in reports of trials included in this review.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of all reports identified by the electronic searches as per the ‘Criteria for considering studies for this review’. The review authors were aware of the report authors, institutions and trial results during this assessment.
We classified each abstract as (a) definitely include, (b) unsure, or (c) definitely exclude. We retrieved full-text articles for abstracts classified as (a) definitely include and (b) unsure. Two review authors independently screened the full-text articles and classified them as either (1) included, (2) awaiting clarification or (3) excluded. A third review author resolved any disagreements at each stage. We contacted the authors of studies classified as (2) awaiting assessment for further clarification. The table of excluded studies lists the details of full-text articles classified by both review authors as excluded.
Methods for future updates
If any randomized or quasi-randomized trials are identified in future updates of this review we will adopt the following methods.
Data extraction and management
Two review authors will independently extract data from the reports of the included trials. We will resolve discrepancies through discussion. We will contact the trial investigators about any missing data. One review author will enter data into RevMan 5.2 and a second review author will verify the entered data.
We will extract the following data from the included trials.
- Participants:
- numbers of participants in the trial, age at onset of SDA and age at initiation of treatment for SDA, duration of stimulus deprivation, cause of stimulus deprivation, visual acuity before treatment, and details about refractive correction;
- concomitant ocular pathology that may limit visual outcome (e.g., coloboma, optic nerve hypoplasia, retinal dystrophy). Data from studies including such participants will be included in subgroup analyses;
- adjustment period to spectacle correction.
Intervention: method of occlusion, regimes, use of CAM or pleoptics.
Outcomes: test(s) used; length of follow-up; whether, when and how compliance was assessed.
Assessment of risk of bias in included studies
Two review authors will independently assess the sources of systematic bias in trials according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will evaluate reports of included trials for the following domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
masking of outcome assessors (detection bias);
incomplete outcome data (attrition bias) - number of participants lost to follow-up and the methods used to account for losses to follow-up in the analyses;
selective outcome reporting (reporting bias);
other sources of bias.
Two review authors will grade each of the risk of bias domains as ‘high risk’; ‘low risk’; or ‘unclear risk’ of bias. A third review author will resolve any disagreements between the first two authors. We will not assess masking of participants and care providers because it is not feasible with interventions for SDA. We will contact authors of reports of included trials for any details that are not described in the published reports. We will use available information to assess the potential for risk of bias whenever the trial authors do not respond within four weeks of our communication.
Measures of treatment effect
For dichotomous outcomes, we will calculate odds ratios for rarer outcomes or risk ratios for more frequent outcomes. We will calculate mean differences for continuous outcomes. We will report 95% confidence intervals (CIs) for all summary effect estimates.
Unit of analysis issues
Patients with SDA mostly present with unilateral disease. Consequently, we expect one eye per individual to be the unit of analysis in trials of interventions for SDA. We will refer to available statistical resources such as the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) or the Cochrane Eyes and Vision Group for advise with any unit of analysis issues in data analysis.
Dealing with missing data
We will contact authors of reports for trials included in our review about any missing data. When the trial authors are unable to provide any details then we will include the trial in our qualitative analysis and omit it from meta-analyses for the outcomes with missing data. We will not impute data, rather we will use the available-case data and note the limitations with this method.
Assessment of heterogeneity
We will examine forest plots for overlap of 95% CIs of effect estimates for visual assessment of heterogeneity between effect estimates of the included trials. The I2 statistic and the Chi2 test for heterogeneity will be calculated.
Data synthesis
If we find no evidence of statistical or clinical heterogeneity across included trials, we will combine the results from the trials in meta-analyses using a fixed-effect model. If there is statistical heterogeneity in the absence of clinical heterogeneity, we will compute a summary measure using a random-effects model. In the case where we find substantial statistical (I2 > 50%) or clinical heterogeneity, we will not combine the study results and instead will present our findings in a tabulated or narrative summary.
Subgroup analysis and investigation of heterogeneity
When sufficient data are available and trials are stratified prior to randomization, the following subgroups will be explored:
participants with and without any co-existing ocular pathology (that might be expected to limit visual prognosis);
participants with stimulus deprivation amblyopia associated with a unilateral congenital cataract versus any other unilateral etiology.
Sensitivity analysis
Sensitivity analyses will be conducted, when appropriate, to determine the size and direction of effect when excluding the following:
outcomes measured on uncrowded vision tests;
studies where any risk of bias item has been graded as ‘high risk’;
unpublished studies or industry-funded studies.
Results
Description of studies
Results of the search
The electronic searches conducted in 2004 identified 799 abstracts and titles of which seven appeared to be randomized controlled trials (RCTs) of interventions for amblyopia. Of these, three trials evaluated conventional occlusion therapy but did not include patients with SDA (Clarke 2003; Holmes 2003a; Repka 2003). There were four studies (Keith 1980; Mehdorn 1981; Nyman 1983; Tytla 1981) on the CAM visual stimulator but after reading the full-texts of the studies and contacting the authors, where necessary, it became apparent that only one trial (Nyman 1983) had included participants with SDA and the data relevant to this review were no longer available. All seven trials were therefore excluded (see table: Characteristics of excluded studies).
Full text copies of 25 additional references were obtained because the initial search information was insufficient to establish whether the studies were eligible for inclusion, either because only the title was available, the abstract was unclear, or the abstract or study was written in a language other than English. After further perusal or translation, all studies were found to be ineligible and were excluded. Reasons for exclusion have been documented (see table: Characteristics of excluded studies).
An updated search was done in November 2007 which yielded an additional 53 reports of studies. One RCT including five patients with SDA was found, but it had investigated the effectiveness of an educational program on the predictors of noncompliance to occlusion therapy (Loudon 2006). Five additional RCTs investigated occlusion therapy (Hertle 2007; Repka 2007; Stankovic 2007; Stewart 2007; Wallace 2006) but these included only strabismic or anisometropic amblyopia, or both. Thus, the search did not identify any new trials which met the inclusion criteria for the review.
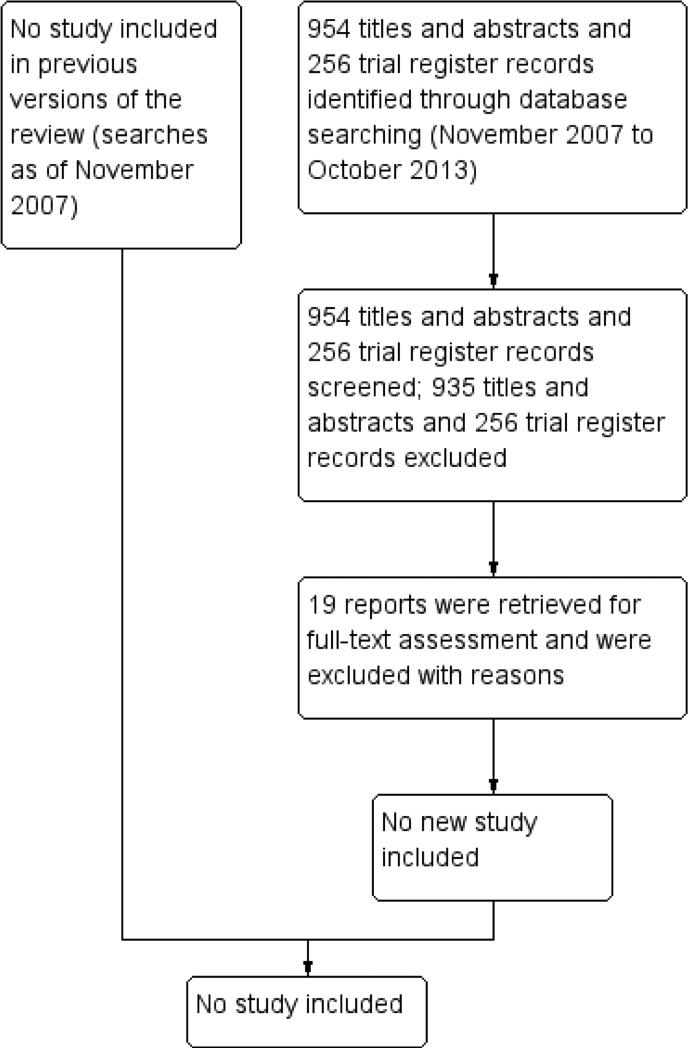
The latest search was conducted in October 2013, which identified 954 additional titles and abstracts and 256 records from trial registers (Figure 1). After review of the records, we assessed 930 titles and abstracts and all trial register records as ’definitely exclude, and 24 as unsure. Five of the 24 references that were assessed as unsure were editorials or commentories that did not report original data and were excluded. We retrieved the remaining 19 full-text articles and further examined their eligibility. We excluded all 19 citations from the review and the reasons for excluding these citations are described in the table Characteristics of excluded studies.
Figure 1.
Results from searching for studies for inclusion in the review.
Risk of bias in included studies
We found no randomized or quasi-randomized trials eligible for inclusion in the review.
Effects of interventions
None of the studies identified in our searches were eligible for inclusion, highlighting a significant gap in existing evidence for the treatment of stimulus deprivation amblyopia (SDA).
Discussion
We did not find any RCT that had evaluated the effects of occlusion or any other treatment for SDA, highlighting a gap in the evidence and the need for rigorous studies to address this question. Because SDA is generally accepted to be more severe and resistant to treatment compared with amblyopia due to other causes, participants with SDA have been excluded from existing RCTs (Kanski 1994; Taylor 1997).
Because of co-existing pathology, the young age of afflicted patients, and limitations of clinical tests, it is difficult to quantify the degree of visual deficit, determine how much of it is attributable to amblyopia, and assess responses to treatment. Success with treatment for SDA is also affected by compliance and the stress for the parents and the child associated with using occlusion therapy.
Existing evidence on the effects of interventions for SDA is derived largely from non-randomized studies of children with SDA caused by unilateral congenital cataract. Although current practice favors aggressive patching in early life, its effectiveness is supported only by a few case-series (Birch 1988; Drummond 1989; Lundvall 2002; Mayer 1989; Robb 1987). Findings from these studies indicate wide variability in the patching regimen employed and the proportion of children who responded to treatment. Less intense occlusion regimens, while being easier to execute, have been advocated because they promote more binocular interaction and stereoacuity. Some of the less intensive occlusion regimens examined in previous studies include occlusion for one hour per day per month of age for the first six months of life (Brown 1999), and patching for six hours and 12 hours a day (Stewart 2007).
Occlusion regimens and treatment outcomes
Current practice generally favors aggressive patching for SDA in early life based on the knowledge that the visual system is much more sensitive to change at this age. Mayer 1989 reported a negative correlation between the number of hours patched and the inter-ocular difference in acuity when treating SDA due to congenital cataract. In non-randomized studies of SDA, the definition of intensive or aggressive patching varied from a minimum of six hours per day to as much as 100% of waking hours. The definition of success also varied across the different studies. Birch 1988 reported that 53% achieved a visual acuity of 20/80 (6/24) or better. Lundvall 2002 found that 20% attained visual acuity of 0.1 (6/7.5) or better, Drummond 1989 reported that 43% achieved better than 20/50 (6/9), and Robb 1987 found 46% achieved visual acuity of at least 20/70 (6/18). This brief summary of some previous studies highlights the different ways in which results can be categorized. These and other dissimilarities in study methodologies make it impossible to meaningfully compare results among these studies. Less intense occlusion regimens are easier to execute and have been advocated because they are expected to promote more binocular interaction and stereoacuity. One study (Brown 1999) reported good visual and binocular results with occlusion of one hour per day per month of age for the first six months of life in children with congenital cataract. Nevertheless, most studies of less intense occlusion regimes have been conducted in children with strabismic or anisometropic amblyopia, or both, and results are probably not generalizable to children with SDA.
Compliance
As with other types of amblyopia, treatment for SDA appears to rely on good compliance in order to achieve a satisfactory outcome. Studies on refractive and strabismic amblyopia have used objective methods to monitor how long occlusion is actually worn (Awan 2005; Loudon 2002; Stewart 2005). These show that the prescribed amount of occlusion is not always achieved. Unfortunately, compliance in treating SDA is often difficult to achieve due to the severity of the visual acuity deficit. While it is not surprising that a treatment that visually compromises a child by means of an adhesive patch is not easy to deliver, justification of adoption or rejection of a treatment must carefully consider evidence of harm alongside evidence of benefit. In a culture where justifying an intervention is increasingly required, the current absence of clear evidence of effectiveness in this area is concerning.
Authors’ Conclusions
Implications for practice
It is not possible to draw reliable conclusions from the data available from non-randomized studies since the study designs either do not compare treatment strategies or are subject to significant bias in the selection of participants for particular treatments. In addition, the variation between studies in treatment delivery and outcome measurement prevents any meaningful comparison or combination of results.
The general trend in practice, based on the proportion of papers we found reporting this treatment, favors an intensive occlusion therapy regimen to attain better visual outcomes. It is important to acknowledge that we did not systematically search for these papers and therefore they may represent a biased sample. It is currently difficult to objectively advise parents or formulate evidence-based guidelines for the management of SDA. Realistic expectations remain uncertain from the treatment for SDA and optimization of treatment regimens.
Implications for research
There is a clear and pressing need for randomized controlled trials (RCTs) to evaluate the effects of interventions for SDA. Occlusion therapy is usually considered the mainstay of treatment for SDA. Some may argue that withholding it from children in a RCT may be unethical. But use of occlusion therapy is not supported by evidence from RCTs, and results in significant stress for both the children and their parents. Because there is a lack of evidence about whether the treatment is effective and safe, it should be ethical to randomize children to no occlusion therapy or an alternative treatment in a trial. It is important to acknowledge that there are practical challenges to setting up a randomized trial for SDA; due to the low numbers, a multicenter study would be required. In addition, many years of follow-up, for example to age seven, would be required to determine long term, post-treatment visual acuity outcomes. Nevertheless, such a study would provide extremely valuable evidence for the management of this condition.
Unsuccessful treatment ultimately results in the same outcome as no treatment, that is, reduced sight in one eye and loss of stereopsis. Exposure to treatment also carries the potential for harm. Thus, future studies on treatment for SDA should report treatment effect and accurately measure any potential physical, emotional or psychological harm.
The following are specific questions that need to be addressed in prospective randomized studies (with appropriate stratification to study any subgroup analyses).
What is the safety and effectiveness of the interventions, including occlusion or patching for SDA?
What is the appropriate duration of treatment for SDA?
What is the level of vision that can realistically be achieved; what are the effects of age at onset and magnitude of visual defect?
What is the optimum occlusion regimen?
What are potential adverse effects from treatments for SDA?
What are the factors associated with satisfactory and unsatisfactory outcomes with various interventions for SDA?
Characteristics Of Studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agafonov 2007 | Not a randomized controlled trial |
| Amiga 1966 | Review, not a clinical trial |
| Botabekova 2004 | Not relevant to stimulus deprivation amblyopia |
| Clarke 2003 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Cramer 1966 | Not a randomized controlled trial |
| Cuppers 1967 | Retrospective case-control study* |
| Fletcher 1969a | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Fletcher 1969b | Retrospective chart review |
| Flynn 1967 | Retrospective chart review |
| Flynn 1968 | Retrospective study |
| Funghini 1973 | Non-comparative study |
| Gernet 1958 | Not relevant to stimulus deprivation amblyopia |
| Hartman 1982 | Not relevant to stimulus deprivation amblyopia |
| Hertle 2007 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Holmes 2003a | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Holmes 2003b | Not relevant to stimulus deprivation amblyopia |
| Iacobucci 1977 | Review article |
| Iturriaga 2012 | Not relevant to stimulus deprivation amblyopia |
| Kamlesh 2008 | Conference abstract; study investigators contacted but no response; unlikely to be relevant to stimulus deprivation amblyopia |
| Kampf 2008 | Not a randomized controlled trial |
| Keith 1980 | Trial of CAM vision stimulator; stimulus deprivation amblyopia not included |
| Kuming 1982 | Before-after study with only 2 participants with stimulus deprivation amblyopia |
| Lang 1965 | Case-series, non-comparative* |
| Lennerstrand 1983 | No participants with stimulus deprivation amblyopia |
| Loudon 2006 | Randomized controlled trial on effectiveness of an educational program on the predictors for noncompliance to occlusion therapy |
| Loudon 2009 | Randomized controlled trial on effectiveness of an educational program on the predictors for noncompliance to occlusion therapy |
| Lyon 2013 | Not relevant to stimulus deprivation amblyopia |
| Mackensen 1965 | Case-series, non-comparative* |
| Malik 1970 | Cohort study |
| Medghalchi 2011 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Mehdorn 1981 | Randomized controlled trial of CAM vision stimulator; stimulus deprivation amblyopia not included |
| Nyman 1983 | Trial of CAM vision stimulator; data on stimulus deprivation amblyopia included but could not be extrapolated and no longer available |
| Pistelka 1973 | Non-comparative study |
| Prakash 1986 | Not relevant to stimulus deprivation amblyopia |
| Priegnitz 1965 | Non-comparative study* |
| Repka 2003 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Repka 2007 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Repka 2010 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Roefs 2008 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Rutstein 2010 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Schor 1983 | Did not include participants with stimulus deprivation amblyopia |
| Shroff 1983 | Non-comparative study |
| Sikder 2008 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Stankovic 2007 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Stewart 2007 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Stojcevska 1975 | Non-comparative study* |
| Thomas 2004 | Not relevant to stimulus deprivation amblyopia |
| Tomlinson 1973 | Non-comparative study |
| Tommila 1969 | Non-comparative study |
| Tommila 1974 | Review article |
| Tytla 1981 | Trial of CAM vision stimulator; stimulus deprivation amblyopia not included |
| Veronneau 1974 | Used historical controls |
| Wallace 2006 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Wallace 2013 | Not relevant to stimulus deprivation amblyopia |
| Walsh 2009 | Randomized controlled trial but stimulus deprivation amblyopia not included |
| Widder 1967 | Non-comparative study* |
| Yan 2008 | Randomized controlled trial but stimulus deprivation amblyopia not included* |
| Zang 1988 | Non-comparative study |
full-text articles of these studies published in non-English languages were reviewed and are noted in this table.
Data And Analyses
This review has no analyses.
What'S New
Last assessed as up-to-date: 28 October 2013.
| Date | Event | Description |
|---|---|---|
| 31 January 2014 | New search has been performed | Issue 2, 2014: Updated searches yielded no new studies. |
| 31 January 2014 | New citation required but conclusions have not changed | Some sections of the review have been updated. The time point for the primary outcome has changed from 6 months to 12 months |
History
Protocol first published: Issue 1, 2005
Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 13 October 2008 | Amended | Converted to new review format. |
| 27 November 2007 | New search has been performed | An update search was done in November 2007; 6 RCTs were excluded but no new trials were included in the review |
| 16 March 2006 | New citation required and conclusions have changed | Substantive amendment |
PLAIN LANGUAGE SUMMARY.
Treatment for amblyopia caused by obstructed vision
Review question
We examined the available evidence regarding occlusion treatment for stimulus deprivation amblyopia (SDA) with respect to vision at the end of treatment.
Background
Amblyopia or ‘lazy eye’ occurs when vision does not develop normally in early childhood. Stimulus deprivation amblyopia (SDA), the type that occurs due to blockage of vision in the eye, for example by a cloudy lens or droopy eyelid, or unequal refractive error in the two eyes (unequal focus of the images, for example one eye is nearsighted and the other eye is farsighted). Eye doctors consider this type of amblyopia to be the most difficult to treat successfully. Although about 1% to 5% of people have some type of amblyopia, SDA is much less common, about 3% of all people with any type of amblyopia. Usually SDA is diagnosed after parents observe a whitish pupil or a droopy eyelid before a baby's first birthday. SDA is often diagnosed after the problem causing these signs is treated and refractive correction (for example, wearing spectacles) is prescribed.
The goal of treatment of SDA is to improve vision in the affected eye and to provide stereopsis, that is, ‘3-D’ vision and depth perception. Treatment may last for several months in order to assure that the affected eye gains as much vision as possible. Also, participation in sports and future employment may be affected by poor vision in one eye or loss of 3-D vision. A common treatment is to occlude or cover the unaffected eye, often with an adhesive patch, in order to force the amblyopic eye to be used. Because young children find occlusion confusing or uncomfortable, occlusion therapy may be difficult for their parents to implement.
Key results
We found no randomized controlled trials (trials in which participants are randomly assigned to one treatment group or another) that evaluated the effectiveness of occlusion therapy for SDA. Thus, well-designed research studies of SDA are needed before we have the information we need to make treatment decisions.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW.
For the 2014 update, we changed the primary outcome time point from six months to 12 months to be consistent with other Cochrane Eyes and Vision Group reviews on amblyopia.
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) devised the search strategies for this review and carried out the electronic searches.
We thank Richard Harrad, Catey Bunce, Sue Elliott and Suzanne Brodney-Folse for their peer review comments throughout the review process. We are also grateful to Milan Mathew for his guidance during the protocol stage and to the CEVG US Project during updates. In addition, we thank Ruthy Acosta for her assistance with screening articles written in Spanish, Qing Pan for her assistance with screening articles written in Chinese, and Kinnar Merchant for assistance with screening articles written in Russian.
Richard Wormald (Co-ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
External sources
• Grant 1 U01 EY020522-01, National Eye Institute, National Institutes of Health, USA.
• Sightsavers International, UK.
• Christian Blind Mission, Germany.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Amblyopia] explode all trees
#2 amblyop* or anopsi* or lazy eye*
#3 MeSH descriptor: [Pupil Disorders] explode all trees
#4 MeSH descriptor: [Cataract] explode all trees
#5 cataract* or pseudoaphakia*
#6 MeSH descriptor: [Blepharoptosis] explode all trees
#7 blepharoptos* or ptosis or ptoses
#8 MeSH descriptor: [Vitreous Hemorrhage] explode all trees
#9 (haemorrhage* or hemorrhage*) near (vitreous)
#10 MeSH descriptor: [Hemangioma, Capillary] explode all trees
#11 (hemangioma* or haemangioma*) near (capillary)
#12 MeSH descriptor: [Aphakia] explode all trees
#13 aphaki*
#14 (stimul* or vision or visual or optical) near (deprivat*)
#15 (scar* or opac* or degenerat*) near (cornea*)
#16 media next opacit*
#17 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #13 or #14 or #15 or #16)
#18 MeSH descriptor: [Sensory Deprivation] explode all trees
#19 patch* or shield*
#20 (stimul* or penalis*) near (optical*)
#21 (stimul* or penalis*) near (vis*)
#22 (therap* or treat* or lens* or complete* or partial*) near (occlus*)
#23 (therap* or treat* or lens* or complete* or partial*) near (pleoptic*)
#24 (#18 or #19 or #20 or #21 or #22 or #23)
#25 (#17 and #24)
Appendix 2. MEDLINE (OvidSP) search strategy
1. Randomized Controlled Trial.pt.
2. Controlled Clinical Trial.pt.
3. (randomized or randomised).ab,ti.
4. placebo.ab,ti.
5. drug therapy.fs.
6. randomly.ab,ti.
7. trial.ab,ti.
8. groups.ab,ti.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp animals/ not humans.sh.
11. 9 not 10
12. exp amblyopia/
13. (amblyop$ or anopsi$ or lazy eye$).tw.
14. exp pupil disorders/
15. exp cataract/
16. (cataract$ or pseudoaphakia$).tw.
17. exp blepharoptosis/
18. (blepharoptos$ or ptosis or ptoses).tw.
19. exp vitreous hemorrhage/
20. ((haemorrhage$ or hemorrhage$) adj3 vitreous).tw.
21. exp hemangioma,capillary/
22. ((hemangioma$ or haemangioma$) adj3 capillary).tw.
23. exp aphakia/
24. aphaki$.tw.
25. ((stimul$ or vision or visual or optical) adj3 deprivat$).tw.
26. ((scar$ or opac$ or degenerat$) adj3 cornea$).tw.
27. (media adj2 opacit$).tw.
28. or/12-27 29. exp sensory deprivation/
30. (patch$ or shield$).tw.
31. ((stimul$ or penalis$) adj3 optical$).tw.
32. ((stimul$ or penalis$) adj3 vis$).tw.
33. ((therap$ or treat$ or lens$ or complete$ or partial$) adj3 occlus$).tw.
34. ((therap$ or treat$ or lens$ or complete$ or partial$) adj3 pleoptic$).tw.
35. or/29-34
36. 28 and 35
37. 11 and 36
The search filter for trials at the beginning of the MEDLINE strategy is based on the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE.com search strategy
#1 ‘randomized controlled trial’/exp
#2 ‘randomization’/exp
#3 ‘double blind procedure’/exp
#4 ‘single blind procedure’/exp
#5 random*:ab,ti
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 ‘animal’/exp OR ‘animal experiment’/exp
#8 ‘human’/exp
#9 #7 AND #8
#10 #7 NOT #9
#11 #6 NOT #10
#12 ‘clinical trial’/exp
#13 (clin* NEAR/3 trial*):ab,ti
#14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti
#15 ‘placebo’/exp
#16 placebo*:ab,ti
#17 random*:ab,ti
#18 ‘experimental design’/exp
#19 ‘crossover procedure’/exp
#20 ‘control group’/exp
#21 ‘latin square design’/exp
#22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
#23 #22 NOT #10
#24 #23 NOT #11
#25 ‘comparative study’/exp
#26 ‘evaluation’/exp
#27 ‘prospective study’/exp
#28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti
#29 #25 OR #26 OR #27 OR #28
#30 #29 NOT #10
#31 #30 NOT (#11 OR #23)
#32 #11 OR #24 OR #31
#33 amblyopia‘/exp
#34 amblyop*:ab,ti OR anopsi*:ab,ti OR (lazy NEAR/1 eye*):ab,ti
#35 ‘pupil disease’/exp
#36 ‘cataract’/exp
#37 cataract*:ab,ti OR pseudoaphakia*:ab,ti
#38 ‘ptosis’/exp
#39 blepharoptos*:ab,ti OR ptosis:ab,ti OR ptoses:ab,ti
#40 ‘vitreous hemorrhage’/exp
#41 ((haemorrhage* OR hemorrhage*) NEAR/3 vitreous):ab,ti
#42 ‘capillary hemangioma’/exp
#43 ((hemangioma* OR haemangioma*) NEAR/3 capillary):ab,ti
#44 ‘aphakia’/exp
#45 aphaki*:ab,ti
#46 ((stimul* OR vision OR visual OR optical) NEAR/3 deprivat*):ab,ti
#47 ((scar* OR opac* OR degenerat*) NEAR/3 cornea*):ab,ti
#48 (media NEAR/2 opacit*):ab,ti
#49 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48
#50 ‘sensory deprivation’/exp
#51 ‘visual deprivation’/exp
#52 patch*:ab,ti OR shield*:ab,ti
#53 ((stimul* OR penalis*) NEAR/3 optical*):ab,ti
#54 ((stimul* OR penalis*) NEAR/3 vis*):ab,ti
#55 ((therap* OR treat* OR lens* OR complete* OR partial*) NEAR/3 occlus*):ab,ti
#56 ((therap* OR treat* OR lens* OR complete* OR partial*) NEAR/3 pleoptic*):ab,ti
#57 #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56
#58 #49 AND #57
#59 #32 AND #58
Appendix 4. LILACS search terms
(Amblyopia OR Ambliopia OR Lazy Eye AND MH:C10.228.140.055$ OR MH:C10.597.751.941.073$ OR MH:C11.966.073$ OR MH:C23.888.592.763.941.073$ OR “Pupil Disorders” OR “Trastornos de la Pupila” OR “Distúrbios Pupilares” OR MH: C10.597.690$ OR MH:C11.710$ OR MH:C23.888.592.708$ OR Cataract$ OR Catarata$ OR Pseudoaphakia$ OR MH: C11.510.245$ OR Blepharoptos$ OR Blefaroptos$ OR MH:C11.338.204$ OR Ptosis OR Ptoses OR “Vitreous Hemorrhage” OR “Hemorragia Vítrea” OR MH:C11.290.960$ OR MH:C23.550.414.756.887$ OR (Hemangioma AND (Capillary OR Capilar)) OR MH:C04.557.645.375.380$ OR Aphakia OR Afaquia OR Afacia OR MH:C11.510.103$ OR ((stimul$ OR vision OR visual OR optical) AND (deprivat$)) OR ((scar$ OR opac$ OR degenerate$) AND (cornea$)) OR (media AND opacity$)) AND (“Sensory Deprivation” OR “Privación Sensorial” OR “Privação Sensorial” OR MH:F02.463.593.696$ OR Patch* OR Shield* OR ((stimul$ OR penalis$) AND (optical$)) OR ((stimul$ OR penalis$) AND (vis$)) OR ((therap$ OR treat$ OR lens$ OR complete$ OR partial$) AND (occlus$)) OR ((therap$ OR treat$ OR lens$ OR complete$ OR partial$) AND (pleoptic$)))
Appendix 5. PubMed search terms
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh])
#2 (amblyop*[tiab] OR anopsi*[tiab] OR lazy eye*[tiab]) NOT Medline[sb]
#3 (cataract*[tiab] OR pseudoaphakia*[tiab]) NOT Medline[sb]
#4 (blepharoptos*[tiab] OR ptosis[tiab] OR ptoses[tiab]) NOT Medline[sb]
#5 ((haemorrhage*[tiab] OR hemorrhage*[tiab]) AND vitreous[tiab]) NOT Medline[sb]
#6 ((hemangioma*[tiab] OR haemangioma*[tiab]) AND capillary[tiab]) NOT Medline[sb]
#7 aphaki*[tiab] NOT Medline[sb]
#8 ((stimulu*[tiab] OR stimulat*[tiab] OR stimuli*[tiab] OR stimulant*[tiab] OR vision[tiab] OR visual[tiab] OR optical[tiab]) AND deprivat*[tiab]) NOT Medline[sb]
#9 ((scar*[tiab] OR opac*[tiab] OR degenerat*[tiab]) AND cornea*[tiab]) NOT Medline[sb]
#10 (media[tiab] AND opacit*[tiab]) NOT Medline[sb]
#11 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 (patch*[tiab] OR shield*[tiab]) NOT Medline[sb]
#13 ((stimulu*[tiab] OR stimulat*[tiab] OR stimuli*[tiab] OR stimulant*[tiab] OR penalis*[tiab]) AND optical*[tiab]) NOT Med-line[sb]
#14 ((stimulu*[tiab] OR stimulat*[tiab] OR stimuli*[tiab] OR stimulant*[tiab] OR penalis*[tiab]) AND vision*[tiab] OR visual*[tiab]) NOT Medline[sb]
#15 ((therapy[tiab] OR therapie*[tiab] OR therapeut*[tiab] OR treat[tiab] OR treated[tiab] OR treatment*[tiab] OR lens*[tiab] OR complete*[tiab] OR partial*[tiab]) AND occlus*[tiab]) NOT Medline[sb]
#16 ((therapy[tiab] OR therapie*[tiab] OR therapeut*[tiab] OR treat[tiab] OR treated[tiab] OR treatment*[tiab] OR lens*[tiab] OR complete*[tiab] OR partial*[tiab]) AND pleoptic*[tiab]) NOT Medline[sb]
#17 #12 OR #13 OR #14 OR #15 OR #16
#18 #11 AND #17
#19 #1 AND #18
Appendix 6 metaRegister of Controlled Trials search strategy
Amblyopia
Appendix 7. ClinicalTrials.gov search strategy
Amblyopia
Appendix 8. ICTRP search strategy
Amblyopia
Footnotes
Citation: Antonio-Santos A, Vedula SS, Hatt SR, Powell C. Occlusion for stimulus deprivation amblyopia. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No.: CD005136. DOI: 10.1002/14651858.CD005136.pub3.
Contributions Of Authors
Conceiving the review: AA
Designing the review: AA
Co-ordinating the review: AA, SSV
Data collection for the review
Designing electronic search strategies: CEVG
Undertaking searches: CEVG
Screening search results: AA, CP, SSV
Organizing retrieval of papers: AA, CP, SSV
Screening retrieved papers against inclusion criteria: AA, CP, SSV, SH
Appraising quality of papers: not applicable
Extracting data from papers: not applicable
Writing to authors of papers for additional information: CEVG
Providing additional data about papers: not applicable
Obtaining and screening data on unpublished studies: CEVG Data management for the review
Entering data into RevMan: not applicable
Analysis of data: not applicable
Interpretation of data
Providing a methodological perspective: SSV, SH
Providing a clinical perspective: AA, CP, SH
Providing a policy perspective: AA, CP, SH
Providing a consumer perspective: AA, CP, SH
Writing the review: AA, SSV. CP, SH
Providing general advice on the review: AA, CP, SH, SSV
Securing funding for the review: not applicable
Performing previous work that was the foundation of the current study: AA, CP, SH
DECLARATIONS OF INTEREST
None known
SOURCES OF SUPPORT
Internal sources
• Michigan State University, Department of Neurology and Ophthalmology, USA.
• Brown University, USA.
• Johns Hopkins University, USA.
INDEX TERMS
Medical Subject Headings (MeSH)
Occlusive Dressings; Amblyopia [etiology; * therapy]; Blepharoptosis [complications]; Cataract [complications]; Treatment Outcome
MeSH check words
Child, Preschool; Humans; Infant
REFERENCES
- Agafonov VV. Development of a technique for ametropia correction with phakic intraocular lenses. Vestnik Rossiiskoi akademii meditsinskikh nauk / Rossiiskaia akademiia meditsinskikh nauk. 2007;(8):52–6. [PubMed] [Google Scholar]
- Arruga A. Occlusion therapy in children. International Ophthalmology Clinics. 1966;6(3):435–52. [PubMed] [Google Scholar]
- Botabekova TK, Kurgambekova NS. A comparative analysis of the efficiency of different methods in the treatment of amblyopia. Vestnik Oftalmologii. 2004;120(5):40–1. [PubMed] [Google Scholar]
- Clarke MP, Wright CM, Hrisos S, Anderson JD, Henderson J, Richardson SR. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ. 2003;327(7426):1251. doi: 10.1136/bmj.327.7426.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer FE, Lamela N, Luqui Lagleyze J, Corsellas A. Use of the electronic flash in the pleoptic treatment of amblyopias [Uso del flash electronico en el tratamiento pleoptico de las ambliopias]. Arquivos Brasileiros de Oftalmologia. 1966;29(3):83–8. [PubMed] [Google Scholar]
- Cuppers C. Effects of pleoptic therapy with special consideration of permanent results. II [Ergebnisse der pleoptischen Therapy unter besonderer Berucksichtigung der Dauerresultate. II]. Documenta Ophthalmologica. 1967;23:570–605. [PubMed] [Google Scholar]
- Fletcher MC, Silverman SJ, Boyd J, Callaway M. Biostatistical studies. Comparison of the management of suppression amblyopia by conventional patching, intensive hospital pleoptics, and intermittent office pleoptics. American Orthoptic Journal. 1969;19:40–7. [PubMed] [Google Scholar]
- Fletcher MC, Abbott W, Girard LJ, Guber D, Silverman SJ, Tomlinson E, et al. Biostatistical studies. Results of biostatistical study of the management of suppression amblyopia by intensive pleoptics versus conventional patching. American Orthoptic Journal. 1969;19:8–30. [PubMed] [Google Scholar]
- Flynn JT, Vereecken E. Effects of pleoptic therapy with special consideration of permanent results. I. Documenta Ophthalmologica. 1967;23:550–69. [PubMed] [Google Scholar]
- Flynn JT. Results with the use of pleoptics in the treatment of amblyopia. Southern Medical Journal. 1968;61(11):1169–71. doi: 10.1097/00007611-196811000-00007. [DOI] [PubMed] [Google Scholar]
- Funghini G. Interest of orthoptic and pleoptic treatment [L'interesse del trattamento ortottico e pleottico]. Minerva Medica. 1973;64(69):3612–5. [PubMed] [Google Scholar]
- Gernet H. Controlled partial occlusion by polarized glasses in treatment of amblyopia and strabismus: preliminary report. Klinische Monatsblätter für Augenheilkunde und für Augenärztliche Fortbildung. 1958;133(3):388–96. [PubMed] [Google Scholar]
- Hartman DK. CAM vision stimulator; a controlled study. New York State Journal of Medicine. 1982;82(5):723–4. [PubMed] [Google Scholar]
- Hertle RW, Scheiman MM, Beck RW, Chandler DL, Bacal DA, Birch E, et al. Stability of visual acuity improvement following discontinuation of amblyopia treatment in children aged 7 to 12 years. Archives of Ophthalmology. 2007;125(5):655–9. doi: 10.1001/archopht.125.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, et al. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110(11):2075–87. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Kraker RT, Cole SR, Repka MX, Birch EE, et al. Impact of patching and atropine treatment on the child and family in the amblyopia treatment study. Archives of Ophthalmology. 2003;121(11):1625–32. doi: 10.1001/archopht.121.11.1625. [DOI] [PubMed] [Google Scholar]
- Iacobucci I. Patching program for treatment of amblyopia. Journal of Pediatric Ophthalmology. 1977;14(1):7–11. [PubMed] [Google Scholar]
- Iturriaga H, Zanolli M, Damm C, Oporto J, Acuna O, Valenzuela F. Frequent evaluation to improve compliance in patients treated with occlusion for amblyopia: a randomized controlled trial. Binocular Vision and Strabismus Quarterly. 2012;27(3):195–204. [PubMed] [Google Scholar]
- Kamlesh GS, Bhola R, Fatima S, Goyal G, Dadeya SC. Levodopa as an adjuvant to conventional occlusion for treatment of amblyopia in children. American Academy of Ophthalmology. 2008 Abstract 176. [Google Scholar]
- Kampf U, Shamshinova A, Kaschtschenko T, Mascolus W, Pillunat L, Haase W. Long-term application of computer-based pleoptics in home therapy: selected results of a prospective multicenter study. Strabismus. 2008;16(4):149–58. doi: 10.1080/09273970802451125. [DOI] [PubMed] [Google Scholar]
- Keith CG, Howell ER, Mitchell DE, Smith S. Clinical trial of the use of rotating grating patterns in the treatment of amblyopia. British Journal of Ophthalmology. 1980;64(8):597–606. doi: 10.1136/bjo.64.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuming BS, Klugman M. The value of CAM visual stimulation. South African Archives of Ophthalmology. 1982;9(3-4):65–70. [Google Scholar]
- Lang J. “Increasing occlusion” in the treatment of amblyopia with eccentric fixation [Die “Ansteigeokklusion” zur Behandlung der Amblyopie mit exzentrischer Fixation]. Ophthalmologica. 1965;149(6):456–62. doi: 10.1159/000304808. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G. Recent advances in the management of amblyopia. International Rehabilitation Medicine. 1983;5(3):128–31. doi: 10.3109/09638288309166946. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Fronius M, Looman CW, Awan M, Simonsz B, van der Maas PJ, et al. Predictors and a remedy for noncompliance with amblyopia therapy in children measured with the occlusion dose monitor. Investigative Ophthalmology and Visual Science. 2006;47(10):4393–400. doi: 10.1167/iovs.05-1428. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Passchier J, Chaker L, de Vos S, Fronius M, Harrad RA, et al. Psychological causes of non-compliance with electronically monitored occlusion therapy for amblyopia. British Journal of Ophthalmology. 2009;93(11):1499–503. doi: 10.1136/bjo.2008.149815. [DOI] [PubMed] [Google Scholar]
- Lyon DW, Hopkins K, Chu RH, Tamkins SM, Cotter SA, Melia BM, et al. Feasibility of a clinical trial of vision therapy for treatment of amblyopia. Optometry and Vision Science. 2013;90(5):475–81. doi: 10.1097/OPX.0b013e31828def04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackensen G, Kroner B, Postic G. On the change of eccentric fixation under occlusion treatment [Zur Anderung der exzentrischen Fixation unter der Okklusionsbehandlung]. Klinische Monatsblätter für Augenheilkunde. 1965;147(2):213–30. [PubMed] [Google Scholar]
- Malik SR, Gupta AK, Grover VK. Occlusion therapy in amblyopia with eccentric fixation. British Journal of Ophthalmology. 1970;54(1):41–5. doi: 10.1136/bjo.54.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medghalchi AR, Dalili S. A randomized trial of atropine vs patching for treatment of moderate amblyopia. Iranian Red Cresecent Medical Journal. 2011;13(8):578–81. [PMC free article] [PubMed] [Google Scholar]
- Mehdorn E, Mattheus S, Schuppe A, Klein U, Kommerell G. Treatment for amblyopia with rotating gratings and subsequent occlusion: a controlled study. International Ophthalmology. 1981;3(3):161–6. doi: 10.1007/BF00130699. [DOI] [PubMed] [Google Scholar]
- Nyman KG, Singh G, Rydberg A, Fornander M. Controlled study comparing CAM treatment with occlusion therapy. Britsh Journal of Ophthalmology. 1983;67(3):178–80. doi: 10.1136/bjo.67.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistelka Z. In-patient pleoptic and orthoptic treatment and its results in pre-school children [Ustavni pleopticko– ortopticka lecba a jeji vysledky u deti predskolniho veku]. Ceskoslovenska Oftalmologie. 1973;29(1):41–9. [PubMed] [Google Scholar]
- Prakash P, Karmacharya PC, Menon V. Evaluation of CAM vision stimulator in the therapy of amblyopia. Indian Journal of Ophthalmology. 1986;34:300–3. [PubMed] [Google Scholar]
- Priegnitz F, Zimmer R. On the occlusive treatment of amblyopia with eccentric fixation [Zur okklusivbehandlung von amblyopien mit exzentrischer fixation]. Das Deutsche Gesundheitswesen. 1965;20(42):1915–8. [PubMed] [Google Scholar]
- Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Archives of Ophthalmology. 2003;121(5):603–11. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- Repka MX, Melia M, Eibschitz-Tsimhoni M, London R, Magoon E, Pediatric Eye Disease Investigator Group The effect on refractive error of unilateral atropine as compared with patching for the treatment of amblyopia. Journal of AAPOS. 2007;11(3):300–2. doi: 10.1016/j.jaapos.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Beck RW, Atkinson SC, Bacal DA, Bremer DL, et al. A pilot study of levodopa as treatment for residual amblyopia in children 8 to 17 years old. Archives of Ophthalmology. 2010;128:1215–7. doi: 10.1001/archophthalmol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roefs AM, Tjiam AM, Vukovic E, Loudon SE, Felius J, Simonsz HJ. Qol and compliance during occlusion therapy for amblyopia in a prospective, double blind study comparing four brands of occlusion patches. Investigative Ophthalmology and Visual Science. 2008 ARVO E-abstract 2583. [Google Scholar]
- Rutstein RP, Quinn GE, Lazar EL, Beck RW, Bonsall DJ, Cotter SA, et al. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010;117(5):998–1004. doi: 10.1016/j.ophtha.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor C, Wick B. Rotating grating treatment of amblyopia with and without eccentric fixation. Journal of the American Optometric Association. 1983;54(6):545–9. [PubMed] [Google Scholar]
- Shroff AP, Billore OP, Dubey AK, Antani PR. A combined approach by spectacle correction, occlusion and active pleoptic treatment in management of amblyopia. Indian Journal of Ophthalmology. 1983;31(5):568–9. [PubMed] [Google Scholar]
- Sikder AM, Husain R, Islam S. A randomized trial of part-time versus full-time patching regimens for treatment of amblyopia. Al-Shifa Journal of Ophthalmology. 2008;4(1):9–18. [Google Scholar]
- Stankovic B, Milenkovic S. Continuous full-time occlusion of the sound eye vs full-time occlusion of the sound eye periodically alternating with occlusion of the amblyopic eye in treatment of amblyopia: a prospective randomized study. European Journal of Ophthalmology. 2007;17(1):11–9. doi: 10.1177/112067210701700103. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ, ROTAS Cooperative Objectively monitored patching regimens for treatment of amblyopia: randomised trial. BMJ. 2007;335(7622):707. doi: 10.1136/bmj.39301.460150.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojcevska V. Pleoptic orthoptic treatment of functional amblyopia [Pleopticko ortopticko lekuvanje na funkcionalnite amblyopii]. Godis en Zbornik na Medicinskiot Fakultet vo Skopje. 1975;21:201–5. [PubMed] [Google Scholar]
- Thomas RB, Rao BV, Matalia J. Comparison of atropine and patching treatments. Ophthalmology. 2004;111(2):407–8. doi: 10.1016/j.ophtha.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Tomlinson E, Jablonski M. Results of modified pleoptic therapy in eccentric fixation. American Orthoptic Journal. 1973;23:60–4. [PubMed] [Google Scholar]
- Tommila V, Nordman E. Late results of pleoptic treatment. British Journal of Ophthalmology. 1969;53(11):769–72. doi: 10.1136/bjo.53.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommila V. Occlusion therapy of amblyopia [Amblyopian peittohoito]. Duodecim. 1974;90(4):295–304. [PubMed] [Google Scholar]
- Tytla ME, Labow-Daily LS. Evaluation of the CAM treatment for amblyopia: A controlled study. Investigative Ophthalmology and Visual Science. 1981;20(3):400–6. [PubMed] [Google Scholar]
- Veronneau Troutman S, Dayanoff SS, Stohler T, Clahane AC. Conventional occlusion vs. pleoptics in the treatment of amblyopia. American Journal of Ophthalmology. 1974;78(1):117–20. doi: 10.1016/0002-9394(74)90019-1. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Pediatric Eye Disease Investigator Group. Edwards AR, Cotter SA, Beck RW, Arnold RW, et al. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113(6):904–12. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Holmes JM, Petersen D, Repka MX, Pediatric Eye Disease Investigator Group A randomized trial of increased patching for amblyopia. Journal of AAPOS. 2013;17(1):e10. [Google Scholar]
- Walsh LA, Hahn EK, LaRoche GR. The method of treatment cessation and recurrence rate of amblyopia. Strabismus. 2009;17(3):107–16. doi: 10.1080/09273970903126709. [DOI] [PubMed] [Google Scholar]
- Widder W. Experiences with occlusion therapy in the 5th--7th years of age [Erfahrungen mit der Okklusionstherapie im 5.–7. Lebensjahr]. Klinische Monatsblätter für Augenheilkunde. 1967;151(5):684–702. [PubMed] [Google Scholar]
- Yan JJ, Peng HC, Wu CX, Liu ZY, Wang YF. A clinical trial of atropine penalization vs patching for treatment of monocular amblyopia. International Journal of Ophthalmology. 2008;8:777–8. [Google Scholar]
- Zang YF, Guo JQ, Liu JQ. Occlusion therapy for amblyopia and stereopsis. Chinese Medical Journal. 1988;101(10):719–22. [PubMed] [Google Scholar]
- American Academy of Ophthalmology, Preferred Practice Pattern: Amblyopia. American Academy of Ophthalmology; San Francisco, Calif: 2002. [Google Scholar]
- Adams GG, Karas MP. Effect of amblyopia on employment prospects. British Journal of Ophthalmology. 1999;83(3):380. doi: 10.1136/bjo.83.3.378c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan M, Proudlock FA, Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Investigative Ophthalmology and Vision Science. 2005;46(4):1435–9. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- Awaya S, Miyake Y, Imaizumi Y, Shinose Y, Kanda T, Komoru K. Amblyopia in man, suggestive of stimulus deprivation amblyopia. Japanese Journal of Ophthalmology. 1973;17:69–82. [Google Scholar]
- Birch EE, Stager DR, Wright WW. Grating acuity development after early surgery for congenital unilateral cataract. Archives of Ophthalmology. 1986;104(12):1783–7. doi: 10.1001/archopht.1986.01050240057040. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager D. Prevalence of good visual acuity following surgery for congenital unilateral cataract. Archives of Ophthalmology. 1988;106(1):40–3. doi: 10.1001/archopht.1988.01060130046025. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Fulton AB. Amblyopia. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. 2nd Edition WB Saunders Co; 2000. [Google Scholar]
- Brown SM, Archer S, Del Monte MA. Stereopsis and binocular vision after surgery for unilateral infantile cataract. Journal of AAPOS. 1999;3(2):109–13. doi: 10.1016/s1091-8531(99)70080-7. [DOI] [PubMed] [Google Scholar]
- Brown SA, Weih LM, Fu CL, Dimitrov P, Taylor HR, McCarty CA. Prevalence of amblyopia and associated refractive errors in an adult population in Victoria, Australia. Ophthalmic Epidemiology. 2000;7(4):249–58. [PubMed] [Google Scholar]
- Buch H, Vinding T, La Cour M, Nielsen NV. The prevalence and causes of bilateral and unilateral blindness in an elderly urban Danish population The Copenhagen City Eye Study. Acta Ophthalmologica Scandinavica. 2001;79(5):441–9. doi: 10.1034/j.1600-0420.2001.790503.x. [DOI] [PubMed] [Google Scholar]
- Dandona R, Dandona L. Childhood blindness in India: a population based perspective. British Journal of Ophthalmology. 2003;87(3):263–5. doi: 10.1136/bjo.87.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray JP, Leibovitch I. Congenital ptosis and amblyopia: a retrospective study of 130 cases. Journal of Pediatric Ophthalmology and Strabismus. 2002;39(4):222–5. doi: 10.3928/0191-3913-20020701-10. [DOI] [PubMed] [Google Scholar]
- Drummond GT, Scott WE, Keech RV. Management of monocular congenital cataracts. Archives of Ophthalmology. 1989;107(1):45–51. doi: 10.1001/archopht.1989.01070010047025. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S, Wybar K. Ocular motility and strabismus. In: Duke-Elder S, editor. System of Ophthalmology. C V Mosby Co; St. Louis, Missouri: 1973. [Google Scholar]
- Enoch J, Hamer R. Image size correction of the unilateral aphakic infant. Ophthalmic Paediatrics and Genetics. 1983;2:153–65. [Google Scholar]
- Fanz RL. Pattern vision in young infants. Psychological Research. 1958;8:43–7. [Google Scholar]
- Ferrone PJ, de Juan E., Jr Vitreous hemorrhage in infants. Archives of Ophthalmology. 1994;112(9):1185–9. doi: 10.1001/archopht.1994.01090210069018. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Manning KA, Dobson V. A behavioral method for efficient screening of visual acuity in young infants. II. Clinical application. Investigative Ophthalmology and Vision Science. 1978;17(12):1151–7. [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Gregg FM, Parks M. Stereopsis after congenital cataract extraction. American Journal of Ophthalmology. 1992;114(3):314–7. doi: 10.1016/s0002-9394(14)71797-0. [DOI] [PubMed] [Google Scholar]
- Gusek-Schneider GC, Martus P. Stimulus deprivation amblyopia in human congenital ptosis: a study of 100 patients. Strabismus. 2000;8(4):261–70. doi: 10.1076/stra.8.4.261.687. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behavioural Brain Research. 1990;41(3):179–98. doi: 10.1016/0166-4328(90)90107-p. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Hillis A, Flynn JT, Hawkins BS. The evolving concept of amblyopia: a challenge to epidemiologists. American Journal of Epidemiology. 1983;118(2):192–205. doi: 10.1093/oxfordjournals.aje.a113627. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Lombroso PJ. Development of the cerebral cortex: IX Cortical development and experience: I. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(9):992–3. doi: 10.1097/00004583-199809000-00021. [DOI] [PubMed] [Google Scholar]
- Kanski JJ. Clinical Ophthalmology. 3rd Edition Butterworth Heinemann; Oxford, UK: 1994. [Google Scholar]
- Kvarnstrom G, Jakobsson P Lennerstrand G. Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmologica Scandinavica. 2001;79(3):240–4. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- Li T, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD006460.pub2. [DOI: 10.1002/14651858.CD006460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon SE, Polling JR, Simonsz HJ. A preliminary report about the relation between visual acuity increase and compliance in patching therapy for amblyopia. Strabismus. 2002;10(2):79–82. doi: 10.1076/stra.10.2.79.8143. [DOI] [PubMed] [Google Scholar]
- Lundvall A, Kugelberg U. Outcome after treatment of congenital unilateral cataract. Acta Ophthalmologica Scandanavica. 2002;80(6):588–92. doi: 10.1034/j.1600-0420.2002.800606.x. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Brent HP. The effects of deprivation on human visual development: studies of children treated for cataracts. In: Morrison FJ, Lord C, Keating DP, editors. Applied Developmental Psychology Vol 3: Psychological Development in Infancy. Academic Press; San Diego, CA: 1989. pp. 39–227. [Google Scholar]
- Mayer DL, Moore B, Robb RM. Assessment of vision and amblyopia by preferential looking tests after early surgery for unilateral congenital cataracts. Journal of Pediatric Ophthalmology and Strabismus. 1989;26(2):61–8. doi: 10.3928/0191-3913-19890301-05. [DOI] [PubMed] [Google Scholar]
- McCulloch DL, Skarf B. Pattern reversal visual evoked potentials following early treatment of unilateral, congenital cataract. Archives of Ophthalmology. 1994;112(4):510–8. doi: 10.1001/archopht.1994.01090160086026. [DOI] [PubMed] [Google Scholar]
- Mein J, Trimble R. Diagnosis and Management of Ocular Motility Disorders. 2nd Edition Blackwell Scientific Publications; Oxford, UK: 1991. [Google Scholar]
- Moseley M, Neufield M, McCarry B, Charnock A, McNamara R, Rice T, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmology and Physiological Optics. 2002;22(4):296–9. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- Newman DK, East MM. Prevalence of amblyopia among defaulters of preschool vision screening. Ophthalmic Epidemiology. 2000;7(1):67–71. [PubMed] [Google Scholar]
- Pratt-Johnson JA, Tillson G. Management of Strabismus and Amblyopia: A Practical Guide. 2nd Edition Thieme; New York, USA: 2001. [Google Scholar]
- Rahi J, Logan S, Timms C, Russell Eggitt I, Taylor D. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. 2002;360(9333):597–602. doi: 10.1016/s0140-6736(02)09782-9. [DOI] [PubMed] [Google Scholar]
- Robb RM, Mayer DL, Moore BD. Results of early treatment of unilateral congenital cataracts. Journal of Pediatric Ophthalmology and Strabismus. 1987;24(4):178–81. doi: 10.3928/0191-3913-19870701-07. [DOI] [PubMed] [Google Scholar]
- Schmidt PP. Vision screening with the RDE stereotest in pediatric populations. Optometry and Vision Science. 1994;71(4):273–81. doi: 10.1097/00006324-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Schulz E. Amblyopia and refractive error in patients who suffered from eyelid haemangioma in early childhood [Deprivationsamblyopie und refraktionsanomalie bei fruhkindlichen lidhamangiomen]. Klinische Monatsblätter für Augenheilkunde. 1982;181(3):192–4. doi: 10.1055/s-2008-1055197. [DOI] [PubMed] [Google Scholar]
- Sebris S, Dobson V, Macdonald M, Teller DY. Acuity cards for visual acuity assessment of infants and children in clinical settings. Clinical Vision Science. 1987;2:45–58. [Google Scholar]
- Simon JW, Parks MM, Price EC. Severe visual loss resulting from occlusion therapy for amblyopia. Journal of Pediatric Ophthalmology and Strabismus. 1987;24(5):244–6. doi: 10.3928/0191-3913-19870901-11. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder A. Defining and measuring treatment outcomes in unilateral amblyopia. British Journal of Ophthalmology. 2003;87(10):1229–31. doi: 10.1136/bjo.87.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ, MOTAS Cooperative Treatment of unilateral amblyopia: factors influencing visual outcome. Investigative Ophthalmology and Vision Science. 2005;46(9):3152–60. doi: 10.1167/iovs.05-0357. [DOI] [PubMed] [Google Scholar]
- Taylor D, Hoyt C. Practical Pediatric Ophthalmology. 1st Edition Blackwell Science; Oxford, UK: 1997. [Google Scholar]
- Taylor K, Elliott S. Interventions for strabismic amblyopia. Cochrane Database of Systematic Reviews. 2011;(Issue 8) doi: 10.1002/14651858.CD006461.pub3. [DOI: 10.1002/14651858.CD006461.pub3] [DOI] [PubMed] [Google Scholar]
- Taylor K, Powell C, Hatt SR. Interventions for unilateral and bilateral refractive amblyopia. Cochrane Database of Systematic Reviews. 2012;(Issue 4) doi: 10.1002/14651858.CD005137.pub3. [DOI: 10.1002/ 14651858.CD005137.pub3] [DOI] [PubMed] [Google Scholar]
- von Noorden GK. Experimental amblyopia in monkeys. Further behavioral observations and clinical correlations. Investigative Ophthalmology. 1973;12(10):721–6. [PubMed] [Google Scholar]
- Von Noorden GK. New clinical aspects of stimulus deprivation amblyopia. American Journal of Ophthalmology. 1981;92(3):416–21. doi: 10.1016/0002-9394(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1963;28(6):1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wright KW, Edelman PM, Walonker F, Yiu S. Reliability of fixation preference testing in diagnosing amblyopia. Archives of Ophthalmology. 1986;104(4):549–53. doi: 10.1001/archopht.1986.01050160105023. [DOI] [PubMed] [Google Scholar]
- Zipf FR. Binocular fixation pattern. Archives of Ophthalmology. 1976;94(3):401–5. doi: 10.1001/archopht.1976.03910030189003. [DOI] [PubMed] [Google Scholar]
- Antonio-Santos A, Mathew M, Powell C, Hatt S. Interventions for stimulus deprivation amblyopia. Cochrane Database of Systematic Reviews. 2004;(Issue 4) doi: 10.1002/14651858.CD005136.pub2. [DOI: 10.1002/14651858.CD005136] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Santos A, Vedula SS, Hatt SR, Powell C. Interventions for stimulus deprivation amblyopia. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD005136.pub2. [DOI: 10.1002/14651858.CD005136.pub2]* Indicates the major publication for the study. [DOI] [PMC free article] [PubMed] [Google Scholar]