Figure 1.
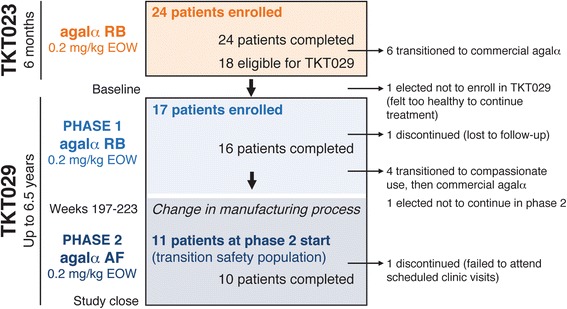
Timeline and flow of patients in the TKT023 core trial and TKT029 extension study. Seventeen patients who completed TKT023 enrolled in TKT029. Screening values from TKT023 week 25/26 were used as TKT029 baseline values. TKT029 was divided into two phases, before and after a change in the agalsidase alfa manufacturing process. Patients were transitioned to phase 2 treatment ~197 to 223 (mean, 210) weeks after phase 1 baseline agalsidase alfa treatment. Only patients who participated in both phases (the transition safety population) were analyzed for this report. EOW, every other week; agalα, agalsidase alfa; RB, roller bottle; AF, animal free.
