Abstract
The majority of human protein-coding genes are predicted to be targets of miRNA-mediated post-transcriptional regulation. The widespread influence of miRNAs is illustrated by their essential roles in all biological processes. Regulated miRNA expression is essential for maintaining cellular differentiation; therefore alterations in miRNA expression patterns are associated with several diseases, including various cancers. High-throughput sequencing technologies revealed low level expressing miRNA isoforms, termed isomiRs. IsomiRs may differ in sequence, length, target preference and expression patterns from their parental miRNA and can arise from differences in miRNA biosynthesis, RNA editing, or SNPs inherent to the miRNA gene. The association between isomiR expression and disease progression is largely unknown. Misregulated miRNA expression is thought to contribute to the formation and/or progression of cancer. However, due to the diversity of targeted transcripts, miRNAs can function as both tumor-suppressor genes and oncogenes as defined by cellular context. Despite this, miRNA profiling studies concluded that the differential expression of particular miRNAs in diseased tissue could aid the diagnosis and treatment of some cancers.
Keywords: Cancer, gene regulation, isomiR, miRNA, miRNA editing, SNP.
INTRODUCTION TO MIRNAS
MicroRNAs (miRNAs) are a family of endogenous RNAs that predominantly regulate the post-transcriptional stages of gene expression. They are single stranded, non-coding RNAs, approximately 21 nucleotides in length. miRNAs were originally identified to be essential for developmental timing [1], however they have subsequently been implicated in the regulation of key biological processes including cell growth, cell differentiation and apoptosis (reviewed in [2]). Within mammalian genomes, miRNAs are predicted to regulate approximately 60% of all coding genes, which illustrates their extensive influence on gene expression [3].
As miRNA-mediated regulation has a considerable impact on numerous pathways, both their function and expression are spatially and developmentally controlled. The miRNA transcriptome is essential for defining and maintaining cellular differentiation and identity. Consequently, compromised miRNA-mediated translational control, for example due to the misregulation of miRNA expression, can result in various diseases including several types of cancer (reviewed in [4-6]).
MIRNA BIOGENESIS
In the human genome, most miRNA coding genes are found either within the introns of protein-coding genes or in non-coding messenger RNAs (mRNAs). A small number of miRNA genes have also been found within the exons of non-coding transcripts [7]. Due to their genomic loci, it is likely that intragenic miRNAs (those encoded within protein-coding genes) share regulatory elements with their host genes [8]. In contrast, independent promoters control the expression of intergenic miRNAs [9].
Human miRNA genes are predominately isolated within the genome, however some are grouped together forming clusters. Clustered miRNAs are transcribed as a single multi-cistronic primary transcript [10, 11], which helps to coordinate the simultaneous regulation of multiple miRNAs. It is common for miRNAs within a cluster to display high sequence homology, and therefore have common mRNA targets [12, 13]. Clusters containing miRNAs with differing target specificities have also been identified and are thought to regulate genes within a particular pathway or protein complex [14, 15].
The location of the miRNA genes may help explain the prevalence of altered miRNA expression in disease as approximately half of annotated human miRNAs are encoded within fragile regions of chromosomes, i.e. areas of the genome known to be associated with human cancers, suggesting that altered miRNA expression has a role in cancer progression [16].
TRANSCRIPTION AND PROCESSING OF MIRNAS
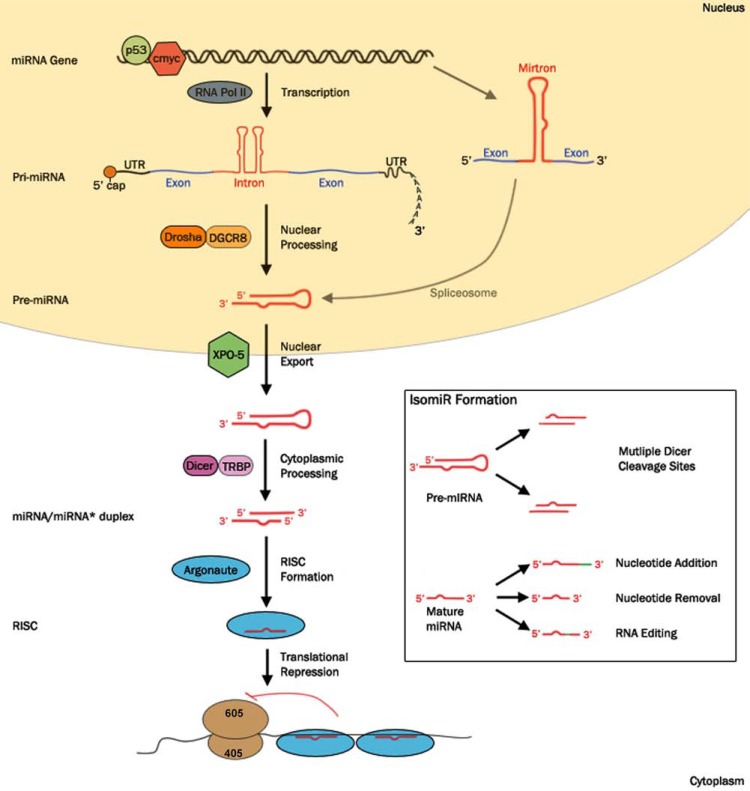
Similar to protein coding genes, most miRNA genes are transcribed by RNA polymerase II forming a capped and polyadenylated RNA precursor or primary transcript (pri-miRNA) (Fig. 1). Transcription of pri-miRNAs is regulated by transcription factors, many of which function only in specific permissive cellular environments. Stress-signals, that are indicative of cancer cells, can regulate the production of particular pri-miRNAs by controlling the functionality of certain transcription factors. For example the tumor suppressor protein p53 [17, 18] and the oncogenic protein c-myc [19] both act to regulate the transcription of miRNA genes with known functions in cancer pathogenesis.
Fig. (1).
miRNA biosynthesis and isomiR formation. Canonical maturation of mammalian miRNAs begins with the miRNA-containing gene being transcribed to form the primary transcript (pri-miRNA). This transcription, a function of RNA polymerase II (RNA Pol II), can be regulated by transcription factors implicated in cancer pathogenesis e.g. p53, c-myc. Pri-miRNA is cleaved by the microprocessor complex (Drosha-DGCR8) to form the precursor miRNA (pre-miRNA) which is then exported into the cytoplasm by Exportin-5 (XPO-5). The protein complex Dicer-TRBP cleaves the pre-miRNA to form the miRNA/miRNA* duplex. One strand of this duplex, the guide strand, is then loaded into an Argonaute protein to form the RNA-Induced Silencing Complex (RISC). The mature miRNA guides the RISC to mRNA targets, resulting in the translation repression of the bound transcripts. Alternative miRNA biosynthesis pathways includes the production of mirtrons, miRNAs that derive from short intronic hairpins, produced from precursors formed by the splicing machinery.
Inset. IsomiRs describe miRNA variants that are produced from a common miRNA gene. Typical sources of isomiRs include multiple Dicer cleavage sites present within the pre-miRNA hairpin. Additionally, isomiRs are formed via post-transcriptional modifications of the mature miRNA, such as the addition or removal of nucleotides, or changing internal nucleotides via RNA editing.
Pri-miRNAs fold into a characteristic stem-loop structure that is recognised by proteins involved in miRNA processing [20]. Within the nucleus, Drosha, an RNAse III enzyme, and DGCR8 (DiGeorge syndrome critical region protein 8), a double stranded RNA binding protein, bind to form the microprocessor complex. The microprocessor cleaves the pri-miRNA to form a precursor (pre)-miRNA. The pre-miRNA is an approximately 70 nucleotide molecule which forms an imperfect hairpin [21]. Pre-miRNAs enter the cytoplasm via the nucleocytoplasmic transport factor Exportin-5 (XPO-5) where further maturation steps occur [22, 23].
Cytoplasmic cleavage of the pre-miRNA hairpin is mediated by Dicer, an RNAse III enzyme, which generates an imperfect miRNA/miRNA* duplex that includes a two nucleotide 3´ overhang [24-26]. The guide strand of the RNA duplex, identified as the strand with the weakest base pairing at its 5´ terminus, is then preferentially incorporated into an Argonaute (Ago) protein [27, 28]. Argonautes are effector proteins required for all miRNA-mediated gene regulation, and an Ago loaded with a miRNA forms the minimal RNA Induced Silencing Complex (RISC) [29, 30].
Alternative miRNA biogenesis pathways have also been identified. For example a minority of miRNAs called mirtrons, coded within the introns of transcripts, are not subjected to Drosha-mediated cleavage before entering the processing pathway [31]. Furthermore, a Dicer independent processing pathway has also been discovered which requires Argonaute-mediated cleavage of the precursor hairpin [32-35].
Each step of the miRNA biogenesis pathway is highly controlled and several proteins implicated in disease, including tumor suppressor and oncogenic proteins, are known to function in regulating this process. These proteins interfere with the processing of all miRNAs or they facilitate and/or inhibit the production of a set of miRNAs. There are also auxiliary protein factors that are specifically regulate the processing of individual miRNAs (reviewed in [36, 37]).
In addition to biogenesis, miRNA half-life and decay is also a controlled process and many different pathways have been found to play a role in miRNA homeostasis [38, 39]. These pathways involve small RNA-degrading nucleases [40], exoribonucleases [41] or decapping scavenger enzymes [42].
MIRNA TARGET RECOGNITION AND FACTORS THAT MODULATE MIRNA ACTION
Mature miRNAs function to guide the RISC to complementary target mRNAs, resulting in the repressed translation of the transcript and/or cleavage of target mRNA. As one miRNA can potentially bind to multiple targets that encode proteins with different functions, a single miRNA can be implicated in diverse cellular pathways and processes.
miRNAs bind to cognate mRNAs with imperfect complementarity. In most cases, the minimal base pairing between a miRNA and the target is determined by a ‘seed’ sequence which is positioned at the 5´ end of the miRNA (nucleotides 2-8) [43-45]. The 3´ proximal nucleotides of the miRNA can potentially enhance target recognition, for example if the seed matching isn’t exact or if the sequence isn’t positioned in an optimal AU-rich context [46, 47].
Thus far, the evidence suggests that the majority of miRNA binding sites implicated in translational repression are located in the 3´ untranslated region (UTR) of the mRNA (reviewed in [2, 48, 49]). However, miRNAs can also bind to target sites found in the 5´ UTR and coding regions of transcripts [50-52].
The majority of experimentally confirmed mRNA targets contain multiple miRNA binding sites, and closely adjacent miRNA binding sites allows for regulation to occur in a cooperative manner [53, 54]. This cooperativity occurs irrespective of the heterogeneous population of miRNAs that may bind a single 3´ UTR [46].
There are several factors which can influence the probability of a miRNA finding its target, including the accessibility of the target mRNA. For example, transcript secondary structure [55] and neighbouring or overlapping RNA-binding motifs could regulate the availability of miRNA binding sites and can therefore modulate the effectiveness of regulation [56-58].
Furthermore, the length of the 3´ UTR of a target can modulate the influence of miRNA-mediated repression. For example, in cancer cells mRNA isoforms with truncated 3´ UTRs, produced from alternative polyadenylation, are highly expressed. The shorter transcripts display differences in function such as altered stability and protein production. These differences are thought to be attributable, in part, to the loss of miRNA-mediated gene regulation [59] .
MECHANISMS OF MIRNA-MEDIATED GENE REGULATION
miRNAs mediate gene regulation via several, non-mutually exclusive mechanisms that target protein synthesis at multiple post-transcriptional stages of gene expression [60, 61]. Many studies conclude that miRNA-mediated inhibition targets the initiation step of translation [62-65], while others indicate the elongation step is affected [66-69]. Additionally, miRNA-mediated mechanisms can promote mRNA deadenylation, degradation and/or mRNA sequestration [70, 71].
These diverse mechanisms are thought to differ due to wide range of potential RISC protein binding partners that act in response to a dynamic cellular environment.
ISOMIRS
Recent advances in sequencing technology has led to the identification of novel miRNAs and the discovery that miRNAs can be heterogeneous in nature varying both in length and sequence [72] (Fig. 1, inset). These miRNA isoforms, or isomiRs [73], interact with both the RISC and translating polysomes, suggesting they bind to target mRNAs [74, 75]. Many of these recently observed miRNA variants are produced at very low levels and display functional redundancies. However some isomiRs display unique expression
patterns which can vary in response to biological stimuli [76-78], or may be indicative of differentiation or disease status of a cell [79, 80], suggesting that isomiR production is dynamically regulated. Although the influence of isomiRs on miRNA-mediated regulation is as yet uncertain, evidence suggests that isomiRs can influence the incorporation of miRNAs into the RISC, miRNA target association, and miRNA stability (reviewed in [81, 82]).
The discovery of miRNA isoforms suggests that one miRNA gene can potentially produce multiple distinct isomiRs. These isoforms can result from variations in the biosynthesis pathway i.e. in the processing of a miRNA precursor molecules. For example pri-miRNAs can be cleaved at multiple sites by Drosha which generates mature miRNAs with differences in their 5’ end [83, 84]. Likewise, the sequence of mature miRNAs is determined by the position of the Dicer cleavage site within the double stranded precursor molecule. However, Dicer can potentially cleave the pre-miRNA at more than one site per precursor molecule, producing multiple miRNAs with distinct seed sequences and target specificity. The location of this cleavage site is influenced by protein binding partners of Dicer [85, 86].
The majority of isomiRs vary in length from the more ubiquitously expressed canonical miRNA. These differences occur either by the subtraction or addition of nucleotides at either the 5´or 3´ end of the miRNA. For instance isomiRs that precisely reflect the parental sequence can be formed due to imprecise cleavage by either Drosha or Dicer or alternatively, by exonucleases removing nucleotides from the end of the miRNA [87, 88]. IsomiRs that do not precisely reflect the parent sequence may also arise via post-transcriptional modifications, which can add extra nucleotides to the mature miRNA [81]. A prevailing modification to mature miRNA molecules is the polyadenylation or uridylation of the 3´ end [72, 89].
In rare cases the internal sequence of an isomiR can vary, and these are termed polymorphic isomiRs. It is possible that these variations are due to single nucleotide polymorphisms (SNPs) within the miRNA gene, however studies have found a relatively low number of SNPs present in miRNAs, suggesting an evolutionary selective pressure against these variants [90, 91].
RNA Editing in IsomiR Formation
Llorens et al. [92] analyzed isomiR production in response to epidermal growth factor (EGF) stimulation as the EGF signaling pathway is correlated with the increased survival of cancer cells [93]. The miRNAs sequenced displayed high sequence heterogeneity but this was not as a result of EGF-mediated mechanisms, however there were cell specific preferences in the isomiRs expressed [92].
Novel miRNAs can also arise as a result of miRNA editing, a process describing post-transcriptional enzymatic modifications to the RNA, which produces miRNAs with single nucleotide mismatches. Kawahara et al. [94] reported that tissue-specific editing within the seed region of a miRNA results in the predominant expression of the edited isoform, which specifically targets different genes.
The misregulation of miRNA editing contributes to cancer progression. Choudhury et al. [95] found that a characteristic of human gliomablastoma cells is a widespread inhibition of miRNA editing. This disruption in mature miRNA seed sequence alterations causes enhanced migration and invasion potential of these cells. A study investigating the role of miRNA editing in metastatic melanoma cells found an RNA editing enzyme was downregulated and consequently alters the miRNA expression profile. However this regulation occurred by targeting miRNA processing and occurred in an RNA-editing independent manner [96]. As yet the importance of misregulated miRNA editing in cancer is not well established.
SNP (SINGLE NUCLEOTIDE POLYMORPHISM) INFLUENCE ON MIRNA MEDIATED GENE REPRESSION
SNPs are the most common form of variation within the human genome [97]. As miRNA-mediated translational repression is dependent on sequence complementarity, it follows that SNPs found in either the miRNA seed regions or in the target site of an mRNA can have a major impact on target identification and subsequent regulation by either hindering effective binding to the target or by the creation of new target sites [4, 98].
Furthermore, the 3´ UTR of a transcript contains numerous regulatory motifs which are required for normal gene expression [99] and many of those motifs which are highly conserved are important to miRNA function [100]. SNPs within these regions could also influence the binding of miRNAs to their targets.
Identifying SNPs that alter miRNA-mediated regulation has been the focus of several studies. Iwai & Naraba [101] found polymorphisms relevant to all stages of miRNA biosynthesis, while Wu et al. [102] found SNPs present in pri-, pre- and mature miRNAs. Moreover, Zeng et al. [103] detected SNPs in the loop sequence of pre-miRNAs which may affect Dicer recognition, and therefore influence the processing of mature miRNAs [104]. Thus SNPs within miRNA genes can potentially affect miRNA expression, maturation and function.
miRNA-related SNPs have been found to correlate to human diseases [105], leading to the formation of databases to catalogue point mutations within miRNAs and their target sites [91, 102, 106], such as miRdSNP [107] and mirSNP [108]. There is a significant association between SNPs found in mature miRNA sequences or in the binding sites of target genes and susceptibility to cancer (reviewed in [4]), which seem specific to particular cancer type [109, 110].
SNPs found within precursor miRNA molecules also correlate to risk of developing disease as shown in recent studies investigating oral cancer [111], lung cancer [112] and chronic lymphocytic leukemia [113].
MIRNA FUNCTION IN CANCER
Established roles of miRNAs include regulating cell growth and tissue differentiation. As both of these processes are deregulated during tumor formation, miRNAs may have a role in the formation and/or progression of cancer (reviewed in [114, 115]).
Lu et al. [116] observed that overall, miRNA expression levels in tumor samples were at lower levels when compared to healthy tissue. This widespread decrease of miRNA expression in cancers is thought to be due to failures in miRNA biosynthesis [117]. Altered miRNA expression can also be the consequences of changes in the levels of proteins involved in the miRNA biosynthesis pathway. While some studies have found a lower level of Dicer and Ago2 (one of the four effector Argonaute proteins in mammalian RISC) [118-120], others revealed no observable change in the expression levels of these proteins [116]. The different miRNA expression profile observed in tumors might therefore be caused by factors that indirectly influence miRNA production.
The relationship between decreased miRNA expression and the loss of cellular differentiation displayed by cancer cells indicates that miRNAs can function as tumor-suppressor genes. Conversely, other studies have shown that the expression of specific miRNAs can be increased in tumor cells and therefore these miRNAs are oncogenes [121-123]. As miRNAs have multiple targets, they have the potential to act as both oncogenic and tumor-suppressors. The specific role of the miRNA is determined by cell type and pattern of expression [124]. Volinia et al. [122] observed that the predicted targets of miRNAs in solid tumor samples comprise a significant proportion of cancer-related genes. However, whether the altered miRNA expression observed in cancer cells is a cause or a consequence of tumor formation remains unclear.
A study investigating human breast cancer cells reported that the decreased expression of a specific family of miRNAs promotes epithelial mesenchymal transition (EMT) [125]. EMT is an essential step in development and cancer progression (reviewed in [126]). Within cancer tissue, there is small population of cells termed cancer stem cells (CSCs). Current hypothesis suggest that CSCs are a main contributor to cancer cell production and cancer progression, and are capable of differentiating into the numerous cell types found in tumors (reviewed in [127, 128]). CSC formation and function is regulated by miRNA-mediated gene regulation [129].
miRNA Profiling in Cancer
As miRNA expression is altered in cancer tissue, profiling of miRNA levels in tumor samples provides an accurate technique of classifying cancer subtypes, and therefore the differential expression of certain miRNAs could aid the diagnosis and treatment of cancer. Data from several profiling studies has shown that miRNA expression pattern is indicative of the differentiation state and the developmental lineage of the tumor [130] and that relatively few miRNAs were required to accurately classify human cancers [116]. In addition, miRNA expression patterns may provide insight into patient survival, further supporting the diagnostic value of miRNA profiling [19, 131]. Recent advances in miRNA expression profiling and their potential as biomarkers are reviewed in [132, 133].
As altered miRNA expression is characteristic of many diseased states, there is also potential use for miRNA profiling in other diseases such as epilepsy [134], multiple sclerosis [135] and coronary artery disease [136].
CONCLUSION
Only five years after the identification of the first human miRNA, it was shown that their expression pattern could faithfully characterize different types of cancers and predict survival rates. Since then, miRNAs are the focal points of biomarker discovery, not only in cancer but also in many other diseases. This is not surprising since miRNAs are central hubs of post-transcriptional gene regulation, due to their ability to regulate multiple target mRNAs. Therefore, changes in miRNA expression levels and target specificity could dramatically change the cellular outcome of gene expression.
miRNA biosynthesis is a multi-stage, tightly regulated process. Increasing number of studies has identified proteins and revealed molecular mechanisms that regulate different steps of miRNA processing and miRNA target recognition. Many of these proteins and cellular pathways have already been associated with disease development before their connection with miRNA mediated gene regulation was discovered. It is very important to understand and further study the regulatory mechanisms and mutations that affect miRNA synthesis and miRNA mediated gene regulation in order to transform miRNAs from established biomarkers to potential drug targets.
ACKNOWLEDGEMENTS
This work was supported by the ARC DP130103027. G.H. is an ARC Future Fellow. We are also grateful to Nham Tran for helpful suggestions.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216(2):671–80. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10(6):389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 6.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–87. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A ):1902–10. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran DL, Pandit KV, Gordon B, Bhattaharjee A, Kaminski N, Benos PV. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS One. 2009;4(4):e5279. doi: 10.1371/journal.pone.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharyya M, Feuerbach L, Bhadra T, Lengauer T, Bandyopadhyay S. MicroRNA transcription start site prediction with multi-objective feature selection. Stat Appl Genet Mol Biol. 2012;11(1):Article 6. doi: 10.2202/1544-6115.1743. [DOI] [PubMed] [Google Scholar]
- 10.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 11.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294(5543):858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 12.Korpal M, Ell BJ, Buffa FM , et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17(9):1101–8. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykxhoorn DM, Wu Y, Xie H , et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One. 2009;4(9):e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sass S, Dietmann S, Burk UC , et al. MicroRNAs coordinately regulate protein complexes. BMC Syst Biol. 2011;5:136. doi: 10.1186/1752-0509-5-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YK, Yu J, Han TS , et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37(5):1672–81. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Ferracin M, Cimmino A , et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 17.Chang TC, Wentzel EA, Kent OAetal. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raver-Shapira N, Marciano E, Meiri Eetal. Transcriptional Activation of miR-34a Contributes to p53-Mediated Apoptosis. Mol Cell. 2007;26(5):731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Dews M, Homayouni A, Yu Detal. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–5. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YS, Nakahara K, Pham JWetal. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117(1):69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Ahn C, Han Jetal. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–9. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 22.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear Export of MicroRNA Precursors. Science. 2004;303(5654):95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DW, Ma E, Shigematsu Hetal. Substrate-specific structural rearrangements of human Dicer. Nat Struct Mol Biol. 2013;20(6):662–70. doi: 10.1038/nsmb.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutvagner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let 7 small temporal RNA. Science. 2001;293(5531):834–8. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 26.Grishok A, Pasquinelli AE, Conte Detal. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C.elegans developmental timing. Cell. 2001;106(1):23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115(2):199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 28.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115(2):209–16. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Carmell MA, Rivas FVetal. Argonaute2 Is the catalytic engine of mammalian RNAi. Science. 2004;305(5689):1437–41. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 30.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15(2):185–97. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–6. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that require Ago catalysis. Nature. 2010;465(7298):584–9. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cifuentes D, Xue H, Taylor DWetal. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328(5986):1694–8. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JS, Maurin T, Robine Netal. Conserved vertebrate mir-451 provides a platform for Dicer-independent Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci USA. 2010;107(34):15163–8. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoda M, Cifuentes D, Izumi Netal. Poly(A)-specific ribonuclease mediates 3'-end trimming of Argonaute2-cleaved precursor microRNAs. Cell Rep. 2013;5(3):715–26. doi: 10.1016/j.celrep.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajan S, Hutvagner G. Another ‘loophole’ in miRNA processing. Mol Cell. 2011;44(3):345–7. doi: 10.1016/j.molcel.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48(1):51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kai ZS, Pasquinelli AE. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat Struct Mol Biol. 2010;17(1):5–10. doi: 10.1038/nsmb.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rüegger S, Großhans H. MicroRNA turnover when how and why. Trends Biochem Sci. 2012;37(10):436–46. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran V, Chen X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science. 2008;321(5895):1490–2. doi: 10.1126/science.1163728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee S, Fasler M, Büssing I, Großhans H. Target-mediated protection of endogenous microRNAs in C.legans. Dev Cell. 2011;20(3):388–96. doi: 10.1016/j.devcel.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Bosse GD, Rüegger S, Ow MCetal. The decapping scavenger enzyme DCS-1 controls microRNA levels in Caenorhabditis elegans. Mol Cell. 2013;50(2):281–7. doi: 10.1016/j.molcel.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 44.Farh KK, Grimson A, Jan C , et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 45.Stark A, Brennecke J, Bushati N, Russel RB, Cohen SM. Animal microRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution. Cell. 2005;123(6):1133–46. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jing Q, Huang S, Guth S , et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120(5):623–34. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 48.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132(1):9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sightκ. Nat Rev Genet. 2008;9(2):102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 50.Hafner M, Landthaler M, Burger L , et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141(1):129–41. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci U S A. 2008;105(39):14879–84. doi: 10.1073/pnas.0803230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5’ UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30(4):460–71. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Broderick JA, Salomon WE, Ryder SP, Aronin N, Zamore PD. Argonaute protein identity and pairing geometry determine cooperativity in mammalian RNA silencing. RNA. 2011;17(10):1858–69. doi: 10.1261/rna.2778911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18(5):504–11. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ameres SL, Martinez J, Schroeder R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007;130(1):101–12. doi: 10.1016/j.cell.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 56.Kedde M, Strasser MJ, Boldajipour B , et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131(7):1273–86. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Elcheva I, Goswami S, Noubissi FK, Spiegelman VS. CRD-BP protects the coding region of beta TrCP1 mRNA from miR-183-mediated degradation. Mol Cell. 2009;35(2):240–6. doi: 10.1016/j.molcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin H, Suh MR, Han J , et al. Human UPF1 participates in small RNA-induced mRNA downregulation. Mol Cell Biol. 2009;29(21):5789–99. doi: 10.1128/MCB.00653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138(4):673–84. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29(1):1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21(3):452–60. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Pillai R, Bhattacharyya SN, Artus CG , et al. Inhibition of translational initiation by Let-7 microRNA in human cells. Science. 2005;309(5740):1573–6. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 63.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–24. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Huang J, Liang Z, Yang B, Tian H, Ma J, Zhang H. Derepression of microRNA-mediated protein translation inhibition by apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) and its family members. J Biol Chem. 2007;282(46):33632–40. doi: 10.1074/jbc.M705116200. [DOI] [PubMed] [Google Scholar]
- 65.Ding XC, Großhans H. Repression of C elegans microRNA targets at the initiation level of translation requires GW182 proteins. EMBO J. 2009;28(3):213–22. doi: 10.1038/emboj.2008.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128(6):1105–18. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J, Krichevsky A, Grad Yetal. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci USA. 2004;101(1):360–5. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson PT, Hatzigeorgiou AG, Mourelatos Z. miRNP mRNA association in polyribosomes in a human neuronal cell line. RNA. 2004;10(3):387–94. doi: 10.1261/rna.5181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jannot G, Bajan S, Giguère NJetal. The ribosomal protein RACK1 is required for microRNA function in both C.elegans and humans. EMBO Rep. 2011;12(6):581–6. doi: 10.1038/embor.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukaya T, Tomari Y. MicroRNAs mediate gene silencing via multiple different pathways in drosophila. Mol Cell. 2012;48(6):825–36. doi: 10.1016/j.molcel.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 71.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 72.Landgraf P, Rusu M, Sheridan Retal. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuchenbauer F, Morin RD, Argiropoulos Betal. In-depth characterization of the microRNA transcriptome in a leukemia progression model. Genome Res. 2008;18(11):1787–97. doi: 10.1101/gr.077578.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee LW, Zhang S, Etheridge Aetal. Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA. 2010;16(11):2170–80. doi: 10.1261/rna.2225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cloonan N, Wani S, Xu Qetal. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol. 2011;12(12):R126. doi: 10.1186/gb-2011-12-12-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wyman SK, Knouf EC, Parkin RKetal. Post-transcriptional generation of miRNA variants by multiple nucleotidyl transferases contributes to miRNA transcriptome complexity. Genome Res. 2011;21(9):1450–61. doi: 10.1101/gr.118059.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burroughs AM, Ando Y, de Hoon MJetal. A comprehensive survey of 3' animal miRNA modification events and a possible role for 3' adenylation in modulating miRNA targeting effectiveness. Genome Res. 2010;20(10):1398–410. doi: 10.1101/gr.106054.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newman MA, Mani V, Hammond SM. Deep sequencing of microRNA precursors reveals extensive 3' end modification. RNA. 2011;17(10):1795–803. doi: 10.1261/rna.2713611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaz C, Ahmad HM, Bharti Retal. Analysis of the microRNA transcriptome and expression of different isomiRs in human peripheral blood mononuclear cells. BMC Res Notes. 2013;6:390. doi: 10.1186/1756-0500-6-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kozubek J, Ma Z, Fleming Eetal. In-Depth characterization of microRNA transcriptome in melanoma. PLoS One. 2013;8(9):e72699. doi: 10.1371/journal.pone.0072699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neilsen CT, Goodall GJ, Bracken CP. IsomiRs the overlooked repertoire in the dynamic microRNAome. Trends Genet. 2012;28(11):544–9. doi: 10.1016/j.tig.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14(8):475–88. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 83.Ma H, Wu Y, Choi JG, Wu H. Lower and upper stem-single-stranded RNA junctions together determine the Drosha cleavage site. Proc Natl Acad Sci USA. 2013;110(51):20687–92. doi: 10.1073/pnas.1311639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu H, Ye C, Ramirez D, Manjunath N. Alternative processing of primary microRNA transcripts by Drosha generates 5' end variation of mature microRNA. PLoS One. 2009;4(10):e7566. doi: 10.1371/journal.pone.0007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013;41(13):6568–76. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukunaga R, Han BW, Hung JH, Xu J, Weng Z, Zamore PD. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell. 2012;151(3):533–46. doi: 10.1016/j.cell.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han BW, Hung JH, Weng Z, Zamore PD, Ameres SL. The 3'-to-5' exoribonuclease Nibbler shapes the 3' ends of microRNAs bound to Drosophila Argonaute1. Curr Biol. 2011;21(22):1878–87. doi: 10.1016/j.cub.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ameres SL, Horwich MD, Hung JHetal. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010;328(5985):1534–9. doi: 10.1126/science.1187058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ebhardt HA, Tsang HH, Dai DC, Liu Y, Bostan B, Fahlman RP. Meta-analysis of small RNA-sequencing errors reveals ubiquitous post-transcriptional RNA modifications. Nucleic Acids Res. 2009;37(8):2461–70. doi: 10.1093/nar/gkp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen K, Rajewsky N. Natural selection on human microRNA binding sites inferred from SNP data. Nature Genet. 2006;38(12):1452–6. doi: 10.1038/ng1910. [DOI] [PubMed] [Google Scholar]
- 91.Saunders MA, Liang H, Li WH. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci USA. 2007;104(9):3300–5. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Llorens F, Hummel M, Pantano Letal. Microarray and deep sequencing cross platform analysis of the miRNome and isomiR variation in response to epidermal growth factor. BMC Genomics. 2013;14:371. doi: 10.1186/1471-2164-14-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways implications for cancer therapy. Cell Signal. 2006;18(12):2089–97. doi: 10.1016/j.cellsig.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315(5815):1137–40. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choudhury Y, Tay FC, Lam DH , et al. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Invest. 2012;122(11):4059–76. doi: 10.1172/JCI62925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nemlich Y, Greenberg E, Ortenberg R , et al. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. J Clin Invest. 2013;123(6):2703–18. doi: 10.1172/JCI62980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frazer KA, Ballinger DG, Cox DR , et al. A second generation human haplotype map of over 3. million SNPs. Nature. 2007;449(7164):851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong J, Tong Y, Zhang HM , et al. Genome-wide identification of SNPs in microRNA genes and SNP effects on microRNA target binding and biogenesis. Hum Mutat. 2012;33(1):254–63. doi: 10.1002/humu.21641. [DOI] [PubMed] [Google Scholar]
- 99.Xie X, Lu J, Kulbokas EJ , et al. Systematic discovery of regulatory motifs in human promoters and 3' UTRs by comparison of several mammals. Nature. 2005;434(7031):338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis BP, Berge CB, Bartel D. Conserved seed pairing often flanked by adenosines indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 101.Iwai N, Naraba H. Polymorphisms in human pre-miRNAs. Biochem Biophys Res Commun. 2005;331(4):1439–44. doi: 10.1016/j.bbrc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 102.Wu M, Jolicoeur N, Li Zetal. Genetic variations of microRNAs in human cancer and their effects on the expression of miRNAs. Carcinogenesis. 2008;29(9):1710–6. doi: 10.1093/carcin/bgn073. [DOI] [PubMed] [Google Scholar]
- 103.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100(17):9779–84. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsutsumi A, Kawamata T, Izumi N, Seitz H, Tomari Y. Recognition of the pre-miRNA structure by Drosophila Dicer-1. Nat Struct Mol Biol. 2011;18(10):1153–8. doi: 10.1038/nsmb.2125. [DOI] [PubMed] [Google Scholar]
- 105.Abelson JF, Kwan KY, ORoak BJetal. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310(5746):317–20. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 106.Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA. Hum Mol Genet. 2007;16(9):1124–31. doi: 10.1093/hmg/ddm062. [DOI] [PubMed] [Google Scholar]
- 107.Bruno AE, Li L, Kalabus JL, Pan Y, Yu A, Hu Z. miRdSNP a database of disease associated SNPs and microRNA target sites on 3'UTR of human genes. BMC Genomics. 2012;13:44. doi: 10.1186/1471-2164-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C, Zhang F, Li Tetal. MirSNP a database of polymorphisms altering miRNA target sites identifies miRNa-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;13:661. doi: 10.1186/1471-2164-13-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li L, Sheng Y, Lv L, Gao J. The association between two microRNA variants (miR-499 miR-149) and gastrointestinal cancer risk a meta-analysis. PLoS One. 2013;8(11):e81967. doi: 10.1371/journal.pone.0081967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Q, Liu H, Xiong Hetal. Polymorphisms at the microRNA binding-site of the stem cell marker gene CD133 modify susceptibility to and survival of gastric cancer. Mol Carcinog. 2013 doi: 10.1002/mc.22113. [DOI] [PubMed] [Google Scholar]
- 111.Roy R, De Sarkar N, Ghose Setal. Genetic variations at microRNA and processing genes and risk of oral cancer. Tumor Biol. 2014;35(4):3401–14. doi: 10.1007/s13277-013-1450-3. [DOI] [PubMed] [Google Scholar]
- 112.Jeon H, Lee YH, Lee SYetal. A common polymorphism in pre-microRNA-146a is associated with lung cancer risk in a Korean population. Gene. 2014;534(1):66–71. doi: 10.1016/j.gene.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 113.Kminkova J, Mraz M, Zaprazna Ketal. Identification of novel sequence variations in microRNAs in chronic lymphocytic leukemia. Carcinogenesis. 2014 doi: 10.1093/carcin/bgt396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11(12):849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Esquela-Kerscher A, Slack FJ. Oncomirs microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 116.Lu J, Getz G, Miska EAetal. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 117.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20(16):2202–7. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karube Y, Tanaka H, Osada Hetal. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96(2):111–5. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Huang J, Yang Netal. MicroRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA. 2006;103(24):9136–41. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Völler D, Reinders J, Meister G, Bosserhoff AK. Strong reduction of AGO2 expression in melanoma and cellular consequences. Br J Cancer. 2013;109(12):3116–24. doi: 10.1038/bjc.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ota A, Tagawa H, Karnan Setal. Identification and characterization of a novel gene C13orf25 as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64(9):3087–95. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 122.Volinia S, Calin GA, Liu CGetal. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He L, Thomson JM, Hemann MTetal. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fabbri M, Ivan M, Cimmino A, Negrini M, Calin GA. Regulatory mechanisms of microRNAs involvement in cancer. Expert Opin Biol Ther. 2007;7(7):1009–19. doi: 10.1517/14712598.7.7.1009. [DOI] [PubMed] [Google Scholar]
- 125.Iliopoulos D, Polytarchou C, Hatziapostolou Metal. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2(92):ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thiery JP, Aclogue H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 127.Lathia JD, Venere M, Rao MS, Rish JN. Seeing is believing are cancer stem cells the Loch Ness monster of tumor biologyκ. Stem Cell Rev. 2011;7(2):227–37. doi: 10.1007/s12015-010-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu HG, Chen C, Yang H, Pan YF, Zhang XH. Cancer stem cell subsets and their relationships. J Transl Med. 2011;9:50. doi: 10.1186/1479-5876-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlia PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39(5):761–72. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He H, Jazdzewski K, Li Wetal. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102(52):19075–80. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yanaihara N, Caplen N, Bowman Eetal. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 132.Iorio MV, Croce CM. MicroRNA dysregulation in cancer diagnostics monitoring and therapeutics A comprehensive review. EMBO Mol Med. 2012;4(3):143–59. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X, Wang Q, Zheng Yetal. Prioritizing human cancer microRNAs based on genes functional consistency between microRNA and cancer. Nucleic Acids Res. 2011;39(22):e153. doi: 10.1093/nar/gkr770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Henshall DC. MicroRNAs in the pathophysiology and treatment of status epilepticus. Front Mol Neurosci. 2013;6:37. doi: 10.3389/fnmol.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fenglio C, Ridolfi E, Cantoni Cetal. Decreased circulating miRNA levels in patients with primary progressive multiple sclerosis. Mult Scler. 2013;19(14):1938–42. doi: 10.1177/1352458513485654. [DOI] [PubMed] [Google Scholar]
- 136.Li K, Zhang T, Fan H , et al. The analysis of microRNA expression profiling for coronary artery disease. Cardiology. 2014;127(1):62–9. doi: 10.1159/000354872. [DOI] [PubMed] [Google Scholar]