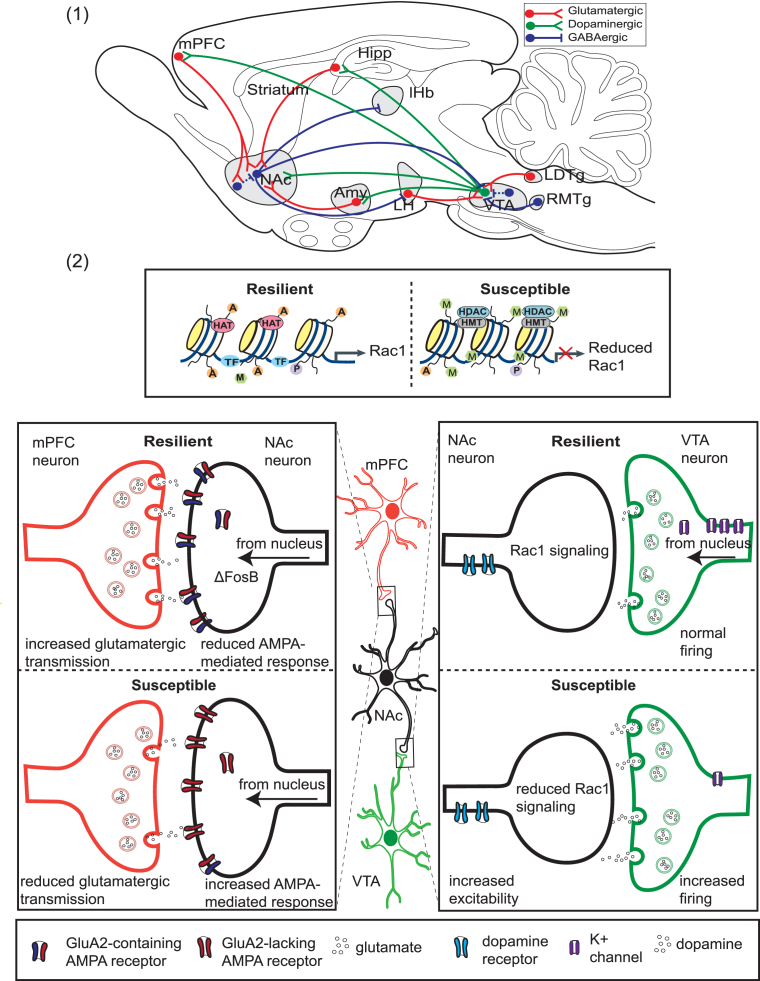
Fig. 3.
Molecular and cellular mechanisms of resilience in the mesocorticolimbic reward circuitry. (1) A simplified schematic of the VTA–NAc circuit, showing the major glutamatergic, GABAergic, and dopaminergic projections to and from the ventral tegmental area (VTA) and nucleus accumbens (NAc) in the rodent. Dashed lines indicate internal, inhibitory connections mediated by interneurons. Hipp, hippocampus; mPFC, medial prefrontal cortex; Amy, amygdala; LH, lateral habenula, RTMg, rostromedial tegmentum; LDTg, lateral dorsal tegmentum. (2) Shown are examples of processes that contribute to resilience vs. susceptibility to chronic social defeat stress. Within the NAc, resilient mice lack the reduced Rac1 gene transcription characteristic of susceptible animals and attributed to reduced histone pan-acetylation (specified by A) and enhanced lysine 27 methylation (specified by M). In susceptible animals, reduced expression of Rac1 leads to actin cytoskeletal reorganization, and an increase in stubby, immature spines. Also in NAc neurons, ΔFosB activity promotes resilience by increasing expression of GluA2. Although shown here for simplicity in the synapse, ΔFosB is a transcription factor, and its activity occurs in the nucleus. GluA2-containing AMPA glutamate receptors are Ca2+ impermeable, reducing AMPA-mediated glutamate response and excitability. In the mPFC, increased glutamatergic transmission has been associated with resilience, and optogenetic stimulation of ChR2 in the mPFC has been shown to promote resilience. In the VTA, resilient animals lack the stress-induced increase in dopaminergic phasic firing that drives susceptibility. Increased firing in susceptible animals is driven by an increased cationic current. Resilient animals homeostatically maintain normal firing rate through the induction of K+ channels. HAT, histone acetyltransferase; HDAC, histone deacetylase; HMT, histone methyltransferase; P, phosphorylation; TF, transcription factor.