Abstract
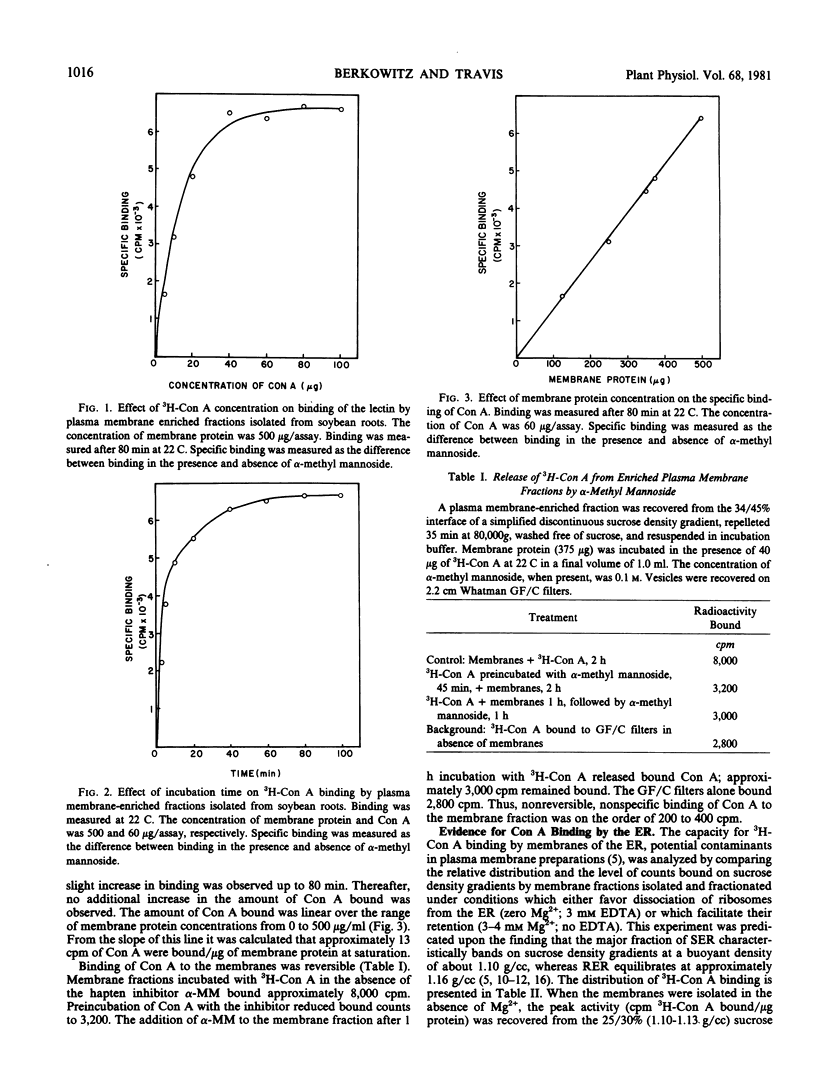
The binding of concanavalin A (Con A) to soybean root membranes in plasma membrane enriched fractions (recovered from the 34/45% interface of simplified discontinuous sucrose density gradients) was studied using a radiochemical assay employing tritiated (3H)-Con A. The effect of lectin concentration, time, and membrane protein concentration on the specific binding of 3H-Con A by the membranes was evaluated. Kinetic analyses showed that Con A will react with membranes in that fraction in a characteristic and predictable manner. The parameters for an optimal and standard binding assay were established. Maximal binding occurred with Con A concentrations in the range of 8 to 16% of the total membrane protein with incubation times greater than 40 min at 22 C. Approximately 1015 molecules of 3H-Con A were bound per microgram of membrane protein at saturation. Binding was reversible. Greater than 92% of the total Con A bound at saturation was released by addition of α-methyl mannoside.
A major peak of 3H-Con A binding was also observed in fractions recovered from the 25/30% interface of a complex discontinuous sucrose density gradient when membranes were isolated in the absence of Mg2+. When high Mg2+ was present in the isolation and gradient media, the peak was shifted to a fraction recovered from the 34/38% sucrose interface. These results suggest that Con A binding sites are also present on membranes of the endoplasmic reticulum. The amount of Con A bound by endoplasmic reticulum membranes was at least twice the amount bound by membranes in plasma membrane enriched fractions when binding was compared on a per unit membrane protein basis. In contrast, mitochondrial inner membranes, which equilibrate at the same density as plasma membranes, had little ability to bind the lectin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz R. L., Travis R. L. An electron microscope comparison of plasma membrane vesicles from meristematic and mature soybean root tissue. Plant Physiol. 1979 Jun;63(6):1191–1197. doi: 10.1104/pp.63.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Kennedy R. M. Adenosine triphosphatase from soybean callus and root cells. Plant Physiol. 1977 Feb;59(2):264–267. doi: 10.1104/pp.59.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Franke W. W., Kartenbeck J. Concanavalin A binding by isolated plasma membranes and endomembranes from liver and mammary gland. FEBS Lett. 1974 Aug 30;44(3):274–278. doi: 10.1016/0014-5793(74)81156-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Browder S. K., Beevers L. Glycosylation of pea cotyledon membranes. Plant Physiol. 1980 Apr;65(4):648–657. doi: 10.1104/pp.65.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Mense R. M., Beevers L. Membrane-associated Glycosyl Transferases in Cotyledons of Pisum sativum: Differential Effects of Magnesium and Manganese Ions. Plant Physiol. 1978 Nov;62(5):766–772. doi: 10.1104/pp.62.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. An assay for labeled lectin binding to cell surfaces. Methods Enzymol. 1974;32:621–625. doi: 10.1016/0076-6879(74)32063-0. [DOI] [PubMed] [Google Scholar]
- Philipp E. I., Franke W. W., Keenan T. W., Stadler J., Jarasch E. D. Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol. 1976 Jan;68(1):11–29. doi: 10.1083/jcb.68.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim J. R., Knight R. H., van Etten J. L. Synthesis of ribonucleic acids during the germination of Rhizopus stolonifer sporangiospores. Dev Biol. 1974 Nov;41(1):137–145. doi: 10.1016/0012-1606(74)90289-9. [DOI] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Characterization of passive ion transport in plasma membrane vesicles of oat roots. Plant Physiol. 1976 Sep;58(3):304–308. doi: 10.1104/pp.58.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Berkowitz R. L. Characterization of Soybean Plasma Membrane during Development: FREE STEROL COMPOSITION AND CONCANAVALIN A BINDING STUDIES. Plant Physiol. 1980 May;65(5):871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]



