Abstract
Body dysmorphic disorder (BDD) is characterized by distressing and often debilitating preoccupations with misperceived defects in appearance. Research suggests that aberrant visual processing may contribute to these misperceptions. This study used two tasks to probe global and local visual processing as well as set shifting in individuals with BDD. Eighteen unmedicated individuals with BDD and 17 non-clinical controls completed two global-local tasks. The embedded figures task requires participants to determine which of three complex figures contained a simpler figure embedded within it. The Navon task utilizes incongruent stimuli comprised of a large letter (global level) made up of smaller letters (local level). The outcome measures were response time and accuracy rate. On the embedded figures task, BDD individuals were slower and less accurate than controls. On the Navon task, BDD individuals processed both global and local stimuli slower and less accurately than controls, and there was a further decrement in performance when shifting attention between the different levels of stimuli. Worse insight correlated with poorer performance on both tasks. Taken together, these results suggest abnormal global and local processing for non-appearance related stimuli among BDD individuals, in addition to evidence of poor set-shifting abilities. Moreover, these abnormalities appear to relate to the important clinical variable of poor insight. Further research is needed to explore these abnormalities and elucidate their possible role in the development and/or persistence of BDD symptoms.
Keywords: Body dysmorphic disorder, Global-local processing, Navon, Embedded figures task, Set-shifting
1. Introduction
Body dysmorphic disorder (BDD) is characterized by preoccupations with perceived defects in physical appearance, which causes substantial distress or functional impairment (American Psychiatric Association., 2013). BDD is an often-severe disorder, in which 25% attempt suicide (Phillips and Menard, 2006) and nearly half are hospitalized during their lifetime (Phillips et al., 2005). Insight varies along a continuum, with 27–60% holding their belief with delusional intensity (Mancuso et al., 2010). The prevalence of BDD in community samples is estimated to be between 0.7% and 2.4% (Koran et al., 2008; Otto et al., 2001; Rief et al., 2006). Although highly debilitating and relatively common, BDD remains under-recognized and under-studied.
BDD is currently classified in the DSM-5 as an obsessive-compulsive related disorder (American Psychiatric Association., 2013). This is in part due to shared phenomenology of obsessive thoughts and compulsive behaviors; in BDD the repetitive and intrusive thoughts about perceived defects in appearance are often obsessional in nature, and individuals engage in repetitive behaviors such as checking or fixing their appearance, which are conceptualized as compulsive (American Psychiatric Association, 2013; Phillips et al., 2010a). There is growing evidence that abnormal visual processing may be an important phenotypic feature in BDD. For example, neuroimaging studies have shown abnormal brain activation patterns when individuals with BDD view faces. Feusner et al. (2007) found greater left hemisphere activation when individuals with BDD viewed others’ faces; this hemispheric imbalance suggests that they rely more on extraction and processing of details. Feusner et al. (2010c) found that individuals with BDD, when viewing images of their face altered to present only low detail (therefore only representing overall stimulus organization) show hypoactivation relative to healthy controls in striate and extrastriate visual cortex. Individuals with BDD also show abnormalities in visual processing systems for low detail images of non-appearance related stimuli (houses) (Feusner et al., 2011).
Visuoperceptual difficulties may also be implicated in BDD individuals’ difficulties with perception of facial emotional expressions (Buhlmann et al., 2006, 2004) and identity recognition of faces with emotional expressions (Feusner et al., 2010a). Additional evidence suggesting an imbalance in local (detail) versus global (holistic) processing in BDD comes from a study of inverted faces. When viewing inverted faces, relative to upright faces, BDD individuals show less slowing of response time relative to controls (Feusner et al., 2010b). This reduced inversion effect suggests a greater reliance on part decomposition and detail processing, for which inversion has less of an effect than for holistic processing (Farah et al., 1995). Another study using inverted faces demonstrated that individuals with BDD relative to healthy controls had enhanced ability to recognize inverted faces (Jefferies et al., 2012). Similar results were not found in a more recent study, although shorter presentation times and different stimuli were used (Monzani et al., 2013). Collectively, these studies largely support a local bias and global impairment for faces among BDD individuals and suggest similar deficits may exist for stimuli unrelated to BDD symptoms.
Findings from neuropsychological studies complement the results of face processing studies, showing that BDD individuals have difficulty in integrating perceptual information, with the resultant reliance on piecemeal, local information rather than on the larger, organizing features. Thus, when asked to copy the Rey–Osterrieth Complex Figure, individuals with BDD add detail to detail; non-clinical controls start with the global elements, which results in superior recall (Deckersbach et al., 2000). A previous study in individuals with OCD showed a similar pattern to the BDD group in this study (Savage et al., 1999). Individuals with BDD also show impairments in executive function tasks of cognitive flexibility, online manipulation, as well as planning and organizing information (Dunai et al., 2010; Hanes, 1998). Spatial memory capacity and visual memory remain intact (Dunai et al., 2010). A study directly comparing individuals with BDD to those with OCD suggests similar abnormalities on performance of most executive planning tasks, with worse performance in the BDD group on accuracy of spatial planning (Labuschagne et al., 2013). These studies and clinical phenomenology suggest that BDD patients have abnormalities in analyzing visual information and abnormalities in executive functioning. It is possible that these abnormalities in information processing may significantly contribute to the symptomatology.
This study investigated whether aberrant visual information processing arises in BDD as the result of a propensity to focus attention at the local level (i.e., local bias), coupled with a deficit in set-shifting – the process of updating and shifting cognitive strategies in response to environmental changes. Previous studies have found evidence for general abnormalities in executive functioning in BDD (Deckersbach et al., 2000; Dunai et al., 2010; Hanes, 1998), although none have specifically examined set-shifting. In addition, no study in BDD has examined set-shifting with respect to global and local visual stimuli, which may be relevant to the phenomenology of perceptual distortions. In the related disorder OCD, most studies show evidence of deficits in set-shifting ((Kuelz et al., 2004; Penades et al., 2005; Rampacher et al., 2010) and see review (Kuelz et al., 2004)). Multiple studies in anorexia nervosa, a related disorder involving body image disturbance, also have found impairments in set-shifting (for review see (Roberts et al., 2007)).
To this end we used two tasks, the embedded figures task (EFT) (Witkin, 1971) and a modified version of the Navon task (Navon, 1977). The EFT consists of a complex figure comprised of smaller “ embedded” figures; participants were required to select the complex figure that contained an embedded target shape. The Navon tasks consists of global letters made out of local letters; participants were required to detect a target letter, either at the global or local level, while ignoring information at the other level. The EFT has not been performed previously in BDD, to our knowledge. The Navon task has been performed (Monzani et al., 2013) although using different stimuli and methodology (see Discussion).
We hypothesized that individuals with BDD would, a) show a local bias by performing faster than control participants on the EFT and on local trials of a Navon task; and b) demonstrate set-shifting abnormalities consisting of shifting attention more slowly relative to controls on the Navon task between target levels and redirecting their attention the slowest when shifting from local to global. A secondary objective was to investigate the relationship between global-local processing and relevant clinical variables. Specifically, we hypothesized that response times on the local and global Navon trials and on the EFT would correlate negatively with BDD symptom severity and degree of insight. Although this has not been researched before, we hypothesized this relationship based on a proposed model that impairments in local and global processing and set-shifting may contribute to visual perceptual distortions, which subsequently lead to more severe symptoms and poorer insight (Li et al., 2013).
2. Method
2.1. Participants
Participants were recruited from the University of California Los Angeles and surrounding communities in three ways: (a) through referrals from mental health providers, dermatologists and cosmetic surgeons, (b) via posted advertisements, and (c) from a website introducing the research.
Eighteen BDD and seventeen control participants of equivalent sex, age, and educational level, all right-handed with visual acuities of at 20/40 or better (corrected or uncorrected), were recruited from the community. Visual acuity was assessed using the Snellen eye chart. Individuals were excluded from the control group if they met DSM-IV criteria for any current or past Axis I psychiatric disorders, as assessed with the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). We excluded control and BDD individuals if they had any current neurological disorder, were pregnant, or had any current medical disorder that may have affected cerebral metabolism.
None were taking psychotropic medications or enrolled in psychotherapy. All participated in a previous fMRI study of own-face processing (Feusner et al., 2010c). The University's Institutional Review Board approved this study. Written informed consent was obtained from all participants. All participants received $50 for their participation in the research.
2.2. Procedures
Screening assessments to determine eligibility of the participants with BDD included the BDD Diagnostic Module (Phillips et al., 1995), a reliable diagnostic module modeled after the DSM-IV. Diagnoses were made by J.D.F., a board-certified psychiatrist with clinical expertise in this population. All BDD participants had preoccupations with perceived facial defects. Comorbid diagnoses were confirmed by structural clinical interviews using the MINI. Although BDD was the primary diagnosis in all cases, some BDD participants also had major depressive disorder (n = 1), generalized anxiety disorder (n = 2), both (n = 4), or dysthymia and generalized anxiety disorder (n = 1).
Participants completed several widely used and validated rating scales, including the 17-item Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1960) and the Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1959). The BDD version of the Yale-Brown Obsessive-Compulsive Scale (BDD-YBOCS) was used to evaluate symptom severity in BDD participants (Phillips et al., 1997). To assess insight and delusionality, we administered the Brown Assessment of Beliefs Scale (BABS) (Eisen et al., 1998). Higher BABS scores index poorer insight (stronger convictions about their appearance being defective and less able to recognize that this concern may be attributable to a mental illness). Upon completion of each task, participants were instructed to, “rate your anxiety on this task” using a Likert-type scale ranging from 0 (none) to 10 (high).
2.2.1. Embedded figures task
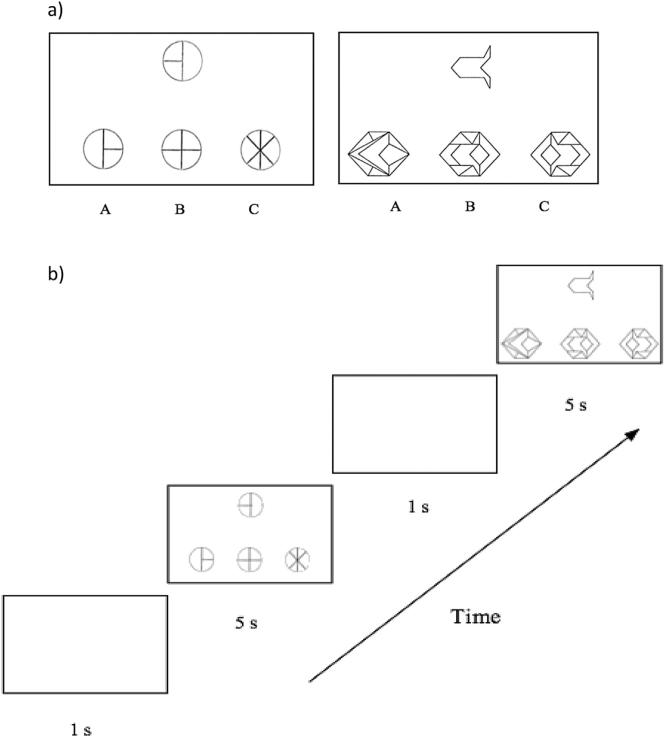
The embedded figures task (EFT) explored global-local processing in the context of matching and extracting simple shapes embedded within complex figures (Oltman et al., 1971). In this paradigm, participants viewed a target shape and three complex figures and were required to detect the complex figure containing the embedded target shape (Witkin, 1971). Response delays and errors may reflect slow processing of local stimuli (Milne and Szczerbinski, 2009). (See Fig. 1 and Supplementary Information.)
Fig. 1.
Stimuli (a) and sequence of events (b) for the embedded figures task. Participants searched for the shape on the top embedded within one of three complex figures on the bottom.
2.2.2. Navon task
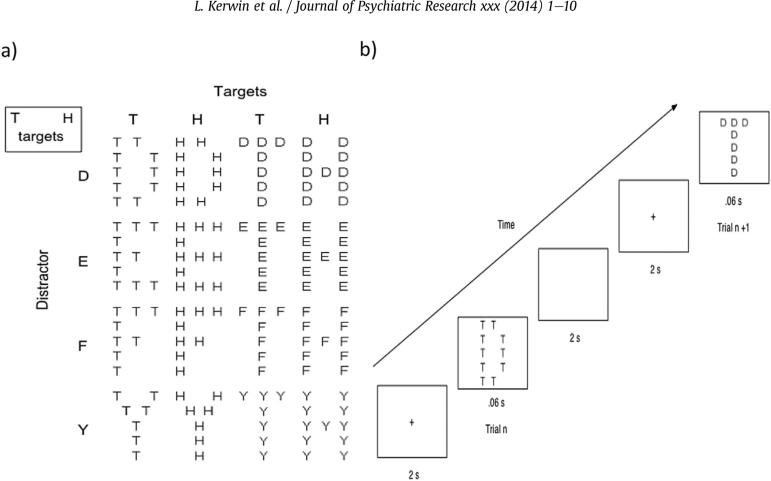
In this paradigm, participants were shown Navon stimuli consisting of a global letter made out of several local letters and were asked to detect a target letter, whether it appears at the global or local level, while ignoring information at the other level. Response delays, errors and interference are believed to occur when the detection of a non-target letter at one level captures or locks the participant's attention despite the attempt to disengage from that level to attend to the letter at the opposite level or initiate response (Navon, 1977).
We utilized a modified version of the Navon task (Yovel et al., 2001), where participants were instructed to identify a target letter (H or T) regardless of whether it occurred on the local or global level. For each trial, a target letter occurred randomly at the global or local level. The task therefore required participants to search and focus on global or local Navon stimuli while ignoring distractors at the other level.
The modified Navon design yielded four set configuration types of interest. Global trials were coded as a global-to-global (non-switch) set when they followed a global trial, and coded as a local-to-global (switch) set when they followed a local trial. Similarly, local trials were coded as a local-to-local (non-switch) set when they followed a local trial and coded as a global-to-local (switch) set when they followed a global trial. (See Fig. 2 and Supplementary Information.)
Fig. 2.
Navon stimuli (a) and sequence of events (b). In each trial one of the two targets appeared at either the global or local level accompanied by one of four distractors at the opposite level.
2.3. Statistical analysis
Statistical analyses were organized into three main sections:
Demographic and clinical differences between BDD and control individuals were analyzed with ANOVA or Chi-square analyses.
Global-local trajectory analyses, in which the manner BDD and control groups performed on the tasks across trials were analyzed with general linear mixed models. Models were fit separately to repeated measures on reaction time (RT) and error rate (with logistic link function) data. Subject served as a random effect, and group and experimental condition (hierarchical target level for one analysis, switch condition for a second analysis, and set configuration for a third analysis) as the fixed effects for the model. For RT analysis, trials were excluded from the analyses if they were missed or incorrect, or if the response occurred after the onset of the next trial. Errors, therefore, included trials in which participants did not respond, responded incorrectly, or responded once the fixation for the subsequent trial appeared. Interactions were decomposed using analysis of simple main effects with Bonferroni corrections for multiple comparisons.
Finally, to test hypotheses about the relationship between symptomatology and performance on the EFT and global and local Navon trials in the BDD group, we used similar general linear mixed models as above with RT as the dependent variable and psychometric scores (BDD-YBOCS and BABS, separately) as covariates. As exploratory analyses, we also tested associations between BDD-YBOCS and BABS, and Navon switch condition and set configuration (global-to-local, local-to-global, local-to-local, or global-to-global trials). As additional exploratory analyses we calculated associations between BDD-YBOCS and BABS, and accuracy rates on the EFT and Navon. We used a significance level of α = 0.05, Bonferroni corrected for multiple comparisons for hypothesized tests (4, for α = 0.0125) and exploratory tests (12, for α = 0.0042).
3. Results
3.1. Sample characterization analyses
Table 1 summarizes the demographic and psychometric data for both groups. There were no significant differences between the BDD and control group in age, gender, or level of education. Typical of this population, all 18 BDD participants had preoccupations with perceived facial defects. The average BDD-YBOCS score in the BDD group was 28.9 ± 5.4 and the average BABS score in the BDD group was 14.6 ± 3.8. Compared to the control group the BDD group had significantly higher mean HAMA and HAMD scores.
Table 1.
Background information for BDD participants and non-clinical controls.
| Controls (n = 17) |
BDD (n = 18) |
||||
|---|---|---|---|---|---|
| Characteristic | Mean | S.D. | Mean | S.D. | Statistics |
| Age (yr) | 28.1 | 5.4 | 28.6 | 6.7 | F1, 33 = 0.04, p = n.s. |
| Education (yr) | 16.8 | 2.3 | 15.5 | 2.7 | F1, 33 = 2.2, p = n.s. |
| Gender | χ2 = 0.03, p = n.s. | ||||
| Male | 7 | 8 | |||
| Female | 10 | 10 | |||
| BDD-YBOCS | 28.9 | 5.4 | |||
| BABS | 14.6 | 3.8 | |||
| HAMD | 1.4 | 1.5 | 10.5 | 7.5 | F1, 33 = 24.4, p < 0.01 |
| HAMA | 1.5 | 1.3 | 12.4 | 8.0 | F1, 33 = 30.3, p < 0.01 |
| Embedded Figures | 1.8 | 1.8 | 3.7 | 2.3 | F1, 27 = 6.7, p < 0.05 |
| Task Anxiety | |||||
| Navon Task Anxiety | 2.6 | 1.4 | 5.0 | 2.7 | F1, 28 = 9.5, p < 0.05 |
BABS = Brown Assessment of Beliefs Scale; BDD = body dysmorphic disorder; BDD-YBOCS = BDD version of the Yale-Brown Obsessive Compulsive Scale; HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale; S.D. = standard deviation.
Of the 18 individuals in the BDD group, 2 did not complete the Navon task and 1 did not complete the EFT task. Thus, 16 BDD individuals completed the Navon task while 17 BDD individuals completed the EFT. Of the 17 in the control group, 1 did not complete the Navon task and 1 did not complete the EFT. Thus, 16 controls completed each task.
Anxiety levels during the task were significantly higher in the BDD than the control group during the EFT and the Navon tasks (Table 1).
3.2. Global-local trajectory analyses
3.2.1. Embedded figures task
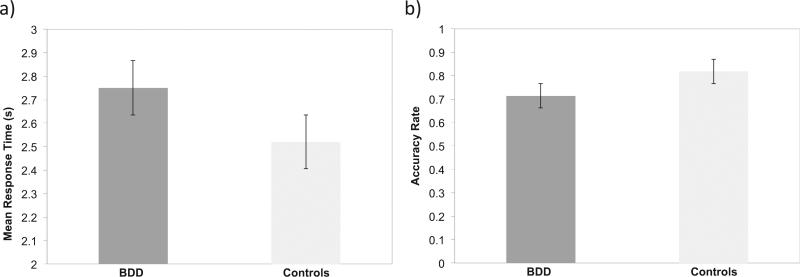
There was a significant main effect of Group on RT (F1 1122 = 4.6, p < 0.05): RT was significantly slower for the BDD than the control group (see Fig. 3a). There was a significant main effect of Group on accuracy rate (F1 1481 = 7.4, p < 0.01, d = 0.84): accuracy was significantly lower in the BDD than the control group (see Fig. 3b).
Fig. 3.
Speed (a) and accuracy (b) in identifying a simple target shape embedded within a complex figure (embedded figure task – EFT) in the body dysmorphic disorder (BDD) and control groups. The BDD group was significantly slower (p < 0.05) and less accurate (p < 0.01) than the control group.
3.2.2. Navon task
3.2.2.1. Hierarchical level
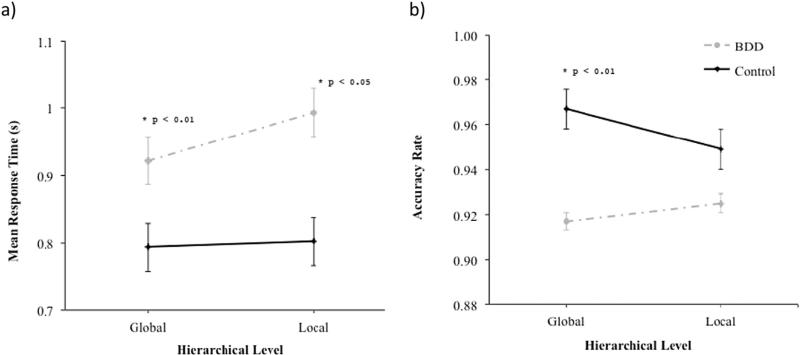
The effects of Group (F1 30 = 10.3, p < 0.01), and Hierarchical Level (F1 3780 = 18.5, p < 0.01), on RT attained statistical significance. In addition, the interaction between Group and Hierarchical Level on RT was significant (F1 3780 = 11.7, p < 0.01) (see Fig. 4a). Pairwise comparisons of the effect of group revealed that the BDD group was slower than controls on local trials (F1 30 = 11.9, p < 0.01, d = 1.25), and global trials (F1 30 = 7.6, p < 0.05, d = 1.0).
Fig. 4.
Speed (a) and accuracy (b) of processing global and local stimuli (Navon task) in the body dysmorphic disorder (BDD) and control groups.
The effect of Group on accuracy attained statistical significance (F1 30 = 6.2, p < 0.05), and there was a significant interaction between Group and Hierarchical Level on accuracy rate (F1 4092 = 4.3, p < 0.05) (see Fig. 4b). Pairwise comparisons of the effect of group within each hierarchical level condition revealed that the BDD group was less accurate than controls on global trials (F1 30 = 6.6, p < 0.01, d = 0.94), but that the groups did not differ on local trials (F1 30 = 3.0, p = N.S., d = 0.58).
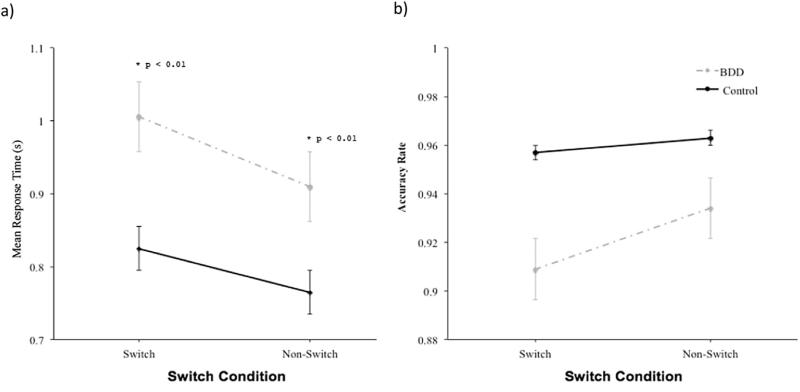
3.2.2.2. Switch condition
The interaction between Group and Switch Condition on RT was significant (F1 3759 = 5.5, p < 0.05) (see Fig. 5a). Pairwise comparisons revealed that the BDD group was slower than controls on switch trials (F1 30 = 12.3, p < 0.01, d = 1.26), and non-switch trials (F1 30 = 7.8, p < 0.01, d = 0.98).
Fig. 5.
Speed (a) and accuracy (b) on the Navon task as a function of maintaining set or switching set between global and local stimuli in the body dysmorphic disorder (BDD) and control groups.
The interaction between Group and Switch Condition on accuracy was not significant (see Fig. 5b).
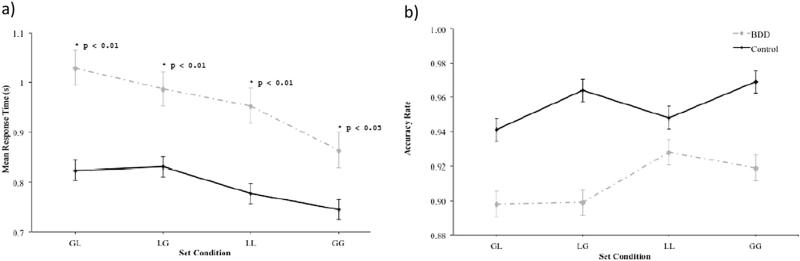
3.2.2.3. Set condition
Considering the four different set configu-ration conditions (global-to-global, local-to-global, local-to-local, or global-to-local), there was a main effect of Group on accuracy (F1 4056 = 5.2, p < 0.05). The interaction between Group and Set Condition on RT was significant (F3 3785 = 5.7, p < 0.001) (see Fig. 6a). Pairwise comparisons revealed that the BDD group's RT was significantly slower than controls on global-to-local trials (F1 3785 = 15.2, p < 0.01, d = 1.37), local-to-global trials (F1 3785 = 8.8, p < 0.01, d = 1.0), local-to-local trials (F1 3785 = 10.7, p < 0.01, d = 1.09), and global-to-global trials (F1 3785 = 5.1, p < 0.05, d = 0.97).
Fig. 6.
Speed (a) and accuracy (b) on the Navon task as a function of set condition (GL – global preceded by local; LG – local preceded by global; LL – local preceded by local; GG – global preceded by global) in the body dysmorphic disorder (BDD) and control groups.
Considering the four different set conditions, the interaction between Group and Set Condition on accuracy rate did not reach statistical significance (F3 4056 = 1.7, p = N.S.) (see Fig. 6b).
3.3. Relationships between BDD clinical variables and task performance
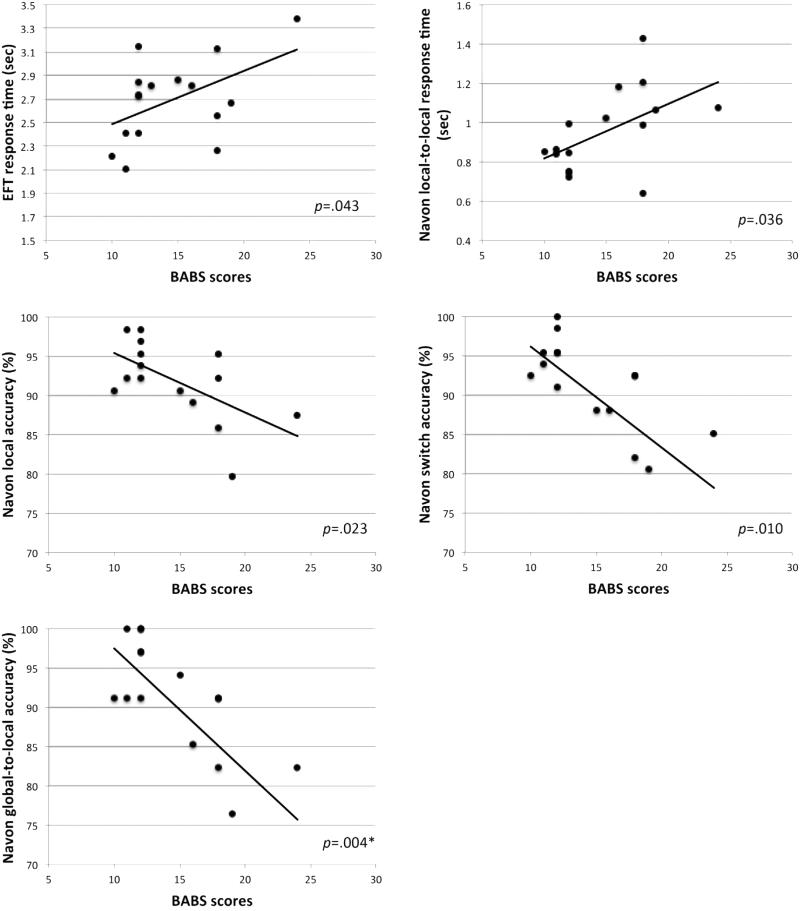
In testing our hypotheses, we found that BABS scores were associated with EFT RT (F1 14.97 = 4.92, p = .043), but results did not survive correction for multiple comparisons. Contrary to our prediction, higher BABS scores (worse insight) were associated with slower response times (see Fig. 7).
Fig. 7.
Scatterplots of BABS scores in relation to response times and accuracy rates for the Navon task and the embedded figures task (EFT). P values are shown for general linear mixed model results. *Surviving Bonferroni correction for multiple comparisons.
For exploratory hypotheses, we found that BABS scores were significantly associated with Navon accuracy on global-to-local trials (F1 14 = 11.90, p = .004). Higher BABS scores (worse insight) were associated with lower accuracy on these conditions. BABS scores were also associated with RT on local-to-local trials (F1 13.94 = 5.40, p = .036), accuracy on local trials (F1 14 = 6.53, p = .023), and accuracy on switch trials (F1 14 = 8.93, p = .010) for the Navon task, but none of these results survived correction for multiple comparisons.
We additionally conducted exploratory post hoc general linear mixed model analyses of both RT and accuracy rates for each task using depression ratings (HAMD scores), anxiety ratings (HAMA scores), and task anxiety as covariates. There were no significant effects of any of these covariates.
4. Discussion
BDD individuals may have impairments in both local and global processing as well as set-shifting for non-appearance-related visual stimuli. Contrary to our hypotheses, the BDD group showed significantly slower (and less accurate) processing of shapes embedded within complex figures, and slower processing of local (as well as global) hierarchical letter stimuli. Also contrary to our hypotheses, there was an inverse relationship between poor insight (but not BDD symptom severity, anxiety, depression, or task-related anxiety), and performance on the Navon and EFT tasks in the BDD group.
Despite the observation that the BDD group did not demonstrate the predicted speed of performance advantages relative to healthy controls, slower response times on local versus global stimuli on the Navon task and slower response times on the EFT may be consistent with a bias for attention to high levels of detail, although associated with slower rather than faster processing. On the Navon task, this may have reflected a tradeoff with accuracy on the local trials, as they were slower than controls but not significantly less accurate. On the EFT the BDD group was slower and less accurate than controls in detecting the local elements separate from their perceptual global contexts. A possible explanation for this is that whereas controls may automatically engage in stimulus-driven bottom-up parallel processing of both global and local elements, BDD individuals, if impaired in global processing, would necessitate top-down attentional shifts that operate on a slower time-scale (Egner and Hirsch, 2005). In other words, the perceptual strategy in BDD may have consisted of piecemeal detail-to-detail scanning of the complex figures whereas the controls may have been aided by a fast global “template” that allows details to be located more easily within it. This would be consistent with a previous study demonstrating that BDD individuals have deficits in visuospatial organization for complex figures (Deckersbach et al., 2000).
On the Navon task we also examined the effect of set-shifting between local and global stimuli. As hypothesized, the BDD group was slower than controls on switch trials (they were also slower than controls on non-switch trials, but the effect was greater for switch trials). Of all the switch and non-switch trials, the BDD group was slowest on global-to-local trials, rather than local-to-global trials as predicted. The modified Navon paradigm most likely probes low-level, perceptually-driven (“bottom-up”) mechanisms more so than conscious, top-down attentional set shifting, due to the rapid presentation time and the fact that stimuli were randomly presented at the global or local hierarchical level. However, because the stimuli were followed by a blank screen for 2 s (rather than being backwardly-masked), it is possible that participants could have utilized top-down mechanisms to determine whether the stimuli was global or local, based on a retinal afterimage (Gazzaley et al., 2005) and/or the image held in visual working memory. If bottom-up mechanisms dominate for this task, then the abnormalities in the BDD group in this study would be consistent with imbalances in early visual pathways responsible for global and local processing. If the top-down mechanisms dominate, this would point to abnormalities in executive functioning, for which other neuropsychological studies have provided evidence (Deckersbach et al., 2000; Dunai et al., 2010; Hanes, 1998). Unfortunately, performance on these tasks (as for many psychophysical tasks) likely requires a mixture of cognitive mechanisms, making it difficult to discern what primarily drives the abnormal performance.
Another possibility is that abnormally slow processing speeds, in general, in those with BDD could explain the findings of slower response times for both the Navon and EFT. If so, this could also secondarily affect accuracy rate on both tasks, since participants only had a limited time to respond. Evidence of slow processing speed in individuals with BDD from previous studies is mixed; slow performance was observed on tasks of executive functioning in neuropsychological studies (Dunai et al., 2010; Hanes, 1998), and for a house-matching task in a previous fMRI study (Feusner et al., 2011), but was not observed in previous studies utilizing face-matching (Feusner et al., 2010b) and face- and simple shape matching (control task) (Feusner et al., 2010c, 2007). Of note, the same BDD individuals and healthy controls in the current study participated in two of the aforementioned studies ((Feusner et al., 2010b) and (Feusner et al., 2010c), except for one BDD and one healthy control participant), and showed no evidence of slower performance.
A recent study used a version of the Navon task to examine the global precedence effect in individuals with BDD and healthy controls (Monzani et al., 2013). The global precedence effect refers to the preference and superiority for processing global aspects of a scene at the expense of the local details and is ascertained by comparing consistent letters, in which the global and local letters share identity (e.g., a large H made of smaller Hs) with inconsistent letters, in which the two levels have different identities (e.g., a large S made of small Hs). The study did not find significantly different performance between groups; the BDD and control groups both showed global precedence (identified global/large and consistent letters faster than the local/small and inconsistent letters). However, their results diverged from results in the current study due to important differences in methodology. They compared inconsistent and consistent stimuli, presented global and local letters separately in blocks, and told participants prior to each block to respond to the global letter or to the local letter. In contrast, in the current study the local and global stimuli were inconsistent, randomly interspersed, and participants searched for specific letters whether at the global or local level. It is possible that individuals with BDD may perform similarly to controls if instructed to focus attention on local or global levels, but do so more slowly when the response is driven by random stimuli, which requires them to quickly and flexibly shift attention.
The observations in the current study of abnormal visual processing of both details and global elements, inaccurate processing of global elements, and reduced flexibility in switching visual attention between global and local features may have significant clinical implications. Those with BDD might spend excessive time fixated on and/or have problems shifting attention away from local information, which coalesces with clinical observations implicating a preoccupation with details in appearance. Slower perceptual processing of local stimuli could lead to explicit awareness of minor flaws that could subsequently exacerbate and maintain BDD symptoms. Thus, such perceptual biases may subsequently contribute to a ruminative and perseverative focus on detailed information. Although our sample size did not permit an analysis of subgroups, future studies may consider investigating whether those with BDD who primarily avoid mirrors show a different or similar pattern of abnormalities, since they are not habitually getting “stuck” in the mirror.
It is interesting to note that the clinical symptom of poor insight, specifically, was associated with slower performance on the EFT, and slower performance and error rates on several Navon conditions. An association between the clinical phenotype of poor insight and disturbed visuospatial perception suggests a model in which insight/delusionality in BDD may be related to impaired visual processing; individuals may have difficulty with or be unable to refute what they visually perceive about their appearance despite being told by others that these appearance “defects” are not noticeable or minor.
There are several limitations to consider. The sample size may have limited the power to detect subtle differences. Several members of the BDD group met DSM-IV criteria for major depression, dysthymia, and generalized anxiety disorder. Major depression (Basso et al., 1996; Gasper and Clore, 2002) or even negative mood (Derryberry and Tucker, 1992) may affect global-local processing independent of BDD pathology. There are fewer reports of the use of global-local tasks in anxiety disorders, but the published findings do not indicate a deficit in this population (Derryberry and Reed, 2002; Sachs et al., 2004). The sample size precluded performing a meaningful comparison of the subgroups with and without these comorbidities. However, the absence of a correlation between depression and anxiety scores and task performance suggests that the behavioral findings in this study are not significantly related, at least dimensionally, to the presence of anxiety or depressive symptoms. Another limitation is that IQ measurements of participants were not available. Thus we cannot rule out the possibility of a general cognitive abnormality that could account for group differences. However, in this study the groups did not significantly differ on total years of education. Moreover, previous neuropsychological studies have not found abnormal IQ in individuals with BDD, nor have they found abnormal performance on all cognitive measures (Deckersbach et al., 2000; Dunai et al., 2010; Hanes, 1998). Finally, diagnostic criteria for this study were based on DSM-IV, yet DSM-5 has since been introduced. DSM-5 includes some changes, most notably the addition of a criterion of repetitive behaviors or mental acts in response to preoccupations (American Psychiatric Association, 2013). The findings from this study are likely still relevant for those meeting DSM-5 criteria, however, as 96% of individuals diagnosed using DSM-IV are estimated to engage in such behaviors or mental acts at any given time (Phillips et al., 2010b).
These results contribute to a nascent body of research that provides a basis for conceptualization of BDD from a cognitive (information processing) perspective (Wilhelm and Neziroglu, 2002), in particular emphasizing the role of a local bias and/or global impairment in the pathophysiology and maintenance of BDD symptomatology. The extent to which a local bias and/or global impairment is influenced by top-down executive functioning with global integration deficits and/or bottom-up perceptual abnormalities is therefore of particular clinical relevance and deserves further study. Regardless of the major driving force, this is potentially modifiable as a treatment strategy. For example, abnormalities in dynamic set-shifting (that is, having to shift in response to randomly-occurring stimuli) found in this study for both global-to-local and local-to-global visual stimuli suggests that a treatment strategy of cognitive remediation aimed at improving attentional shifts in both directions may be useful to test. For example, a perceptual/attentional retraining method could be developed that involves feedback from an eye tracking device. Local level attention requires multiple successions of short fixation durations and saccades, while global level attention involves fewer but longer individual fixations. One could test such retraining using face stimuli and real-time feedback, with instructions for patients to shift between the local level using short, multiple fixations and the global level using long fixation durations (e.g. on the eyes).
A potential impact of this study, and other similar lines of research, is to identify functional abnormalities that may serve as targets for interventions to address the process of thinking rather than the content. To this end, future research may wish to extend the current findings by supplementing behavioral performance indices of attentional bias with convergent measures of overt orienting and brain activity. For example, concurrent eye tracking and event-related electroencephalographic (EEG) potentials could help clarify the time course of attention allocation to global-local stimuli in BDD individuals. In addition, integration of similar paradigms with functional neuroimaging could further delineate the neural systems involved in perception of, and selective attention to, global-local stimuli.
Supplementary Material
Acknowledgments
We would like to thank Susan Bookheimer, Ph.D. for her contributions to the study design and for providing the embedded figures task, and Catherine Sugar, Ph.D. for additional statistical consultation.
Funding source
Funding for this study was provided by the National Institute of Mental Health (5K23MH079212 and R01MH093535 to Dr. Feusner).
Footnotes
Contributors
Dr. Feusner designed the study and was involved in participant recruitment, assessment, and data collection. Dr. Kerwin conceived of the data analysis strategy, in collaboration with Dr. Feusner and with statistical consultation from Dr. Helleman. Dr. Kerwin wrote the first draft of the manuscript, and Drs. Hovav and Feusner provided significant contributions to the final draft including additional data analysis. All authors contributed to and have approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to report.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2014.06.003.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Basso MR, Schefft BK, Ris MD, Dember WN. Mood and global-local visual processing. J Int Neuropsychol Soc. 1996;2:249–55. doi: 10.1017/s1355617700001193. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff N, Wilhelm S. Emotional recognition bias for contempt and anger in body dysmorphic disorder. J Psychiatr Res. 2006;40:105–11. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally R, Etcoff N, Tuschen-Caffier B, Wilhelm S. Emotion recognition deficits in body dysmorphic disorder. J Psychiatr Res. 2004;38:201–6. doi: 10.1016/s0022-3956(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, et al. Characteristics of memory dysfunction in body dysmorphic disorder. J Int Neuropsychol Soc. 2000;6:673–81. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111:225–36. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Tucker DM. Neural mechanisms of emotion. J Consult Clin Psychol. 1992;60:329–38. doi: 10.1037//0022-006x.60.3.329. [DOI] [PubMed] [Google Scholar]
- Dunai J, Labuschagne I, Castle DJ, Kyrios M, Rossell SL. Executive function in body dysmorphic disorder. Psychol Med. 2010;40:1541–8. doi: 10.1017/S003329170999198X. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–90. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The brown assessment of beliefs scale: reliability and validity. Am J Psychiatry. 1998;155:102–8. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995;21:628–34. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Bystritsky A, Hellemann G, Bookheimer S. Impaired identity recognition of faces with emotional expressions in body dysmorphic disorder. Psychiatry Res. 2010a;179:318–23. doi: 10.1016/j.psychres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychol Med. 2011:1–13. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moller H, Altstein L, Sugar C, Bookheimer S, Yoon J, et al. Inverted face processing in body dysmorphic disorder. J Psychiatr Res. 2010b;44:1088–94. doi: 10.1016/j.jpsychires.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H, et al. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry. 2010c;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry. 2007;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: mood and global versus local processing of visual information. Psychol Sci. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–17. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes K. Neuropsychological performance in body dysmorphic disorder. J Int Neuropsychol Soc. 1998;4:167–71. doi: 10.1017/s1355617798001672. [DOI] [PubMed] [Google Scholar]
- Jefferies K, Laws KR, Fineberg NA. Superior face recognition in body dysmorphic disorder. J Obsessive-Compulsive Relat Disord. 2012;1:175–9. [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr. 2008;13:316–22. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Rossell SL, Dunai J, Castle DJ, Kyrios M. A comparison of executive function in Body Dysmorphic Disorder (BDD) and Obsessive-Compulsive Disorder (OCD). J Obsessive-Compulsive Relat Disord. 2013;2:257–62. [Google Scholar]
- Li W, Arienzo D, Feusner JD. Body dysmorphic disorder: neurobiological features and an updated model. Z für Klin Psychol Psychother. 2013;42:184–91. doi: 10.1026/1616-3443/a000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Compr Psychiatry. 2010;51:177–82. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Milne E, Szczerbinski M. Global and local perceptual style, field-independence, and central coherence: an attempt at concept validation. Adv Cogn Psychol. 2009;5:1–26. doi: 10.2478/v10053-008-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani B, Krebs G, Anson M, Veale D, Mataix-Cols D. Holistic versus detailed visual processing in body dysmorphic disorder: testing the inversion, composite and global precedence effects. Psychiatry Research. 2013 doi: 10.1016/j.psychres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: the precedence of global features in visual perception. Cogn Psychol. 1977;9:353–83. [Google Scholar]
- Oltman PK, Raskin E, Witkin HA, Karp SA, Konstadt N. Group embedded figures test. Consulting Psychologists Press; Palo Alto, Calif: 1971. [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry. 2001;158:2061–3. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Andres S, Salamero M, Gasto C. Executive function and nonverbal memory in obsessive-compulsive disorder. Psychiatry Res. 2005;133:81–90. doi: 10.1016/j.psychres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Atala KD, Pope HG., Jr . American Psychiatric Association 148th Annual Meeting. Miami, FL.: 1995. Diagnostic instruments for body dysmorphic disorder. p. 157. [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, Menard W. Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry. 2006;163:1280–2. doi: 10.1176/appi.ajp.163.7.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005;46:317–25. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Stein DJ, Rauch SL, Hollander E, Fallon BA, Barsky A, et al. Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depress Anxiety. 2010a;27:528–55. doi: 10.1002/da.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Wilhelm S, Koran LM, Didie ER, Fallon BA, Feusner J, et al. Body dysmorphic disorder: some key issues for DSM-V. Depress Anxiety. 2010b;27:573–91. doi: 10.1002/da.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampacher F, Lennertz L, Vogeley A, Schulze-Rauschenbach S, Kathmann N, Falkai P, et al. Evidence for specific cognitive deficits in visual information processing in patients with OCD compared to patients with unipolar depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:984–91. doi: 10.1016/j.pnpbp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychol Medicine. 2006;36:877–85. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychol Med. 2007;37:1075–84. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- Sachs G, Anderer P, Dantendorfer K, Saletu B. EEG mapping in patients with social phobia. Psychiatry Res. 2004;131:237–47. doi: 10.1016/j.pscychresns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry. 1999;45:905–16. doi: 10.1016/s0006-3223(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Wilhelm S, Neziroglu F. Cognitive model of body dysmorphic disorder. In: Frost R, Steketee G, editors. Cognitive approaches to obsessions and compulsions theory, assessment, and treatment. Amsterdam: Pergamon: 2002. pp. 203–14. [Google Scholar]
- Witkin HA. A manual for the embedded figures tests. Consulting Psychologists Press; Palo Alto, Calif: 1971. [Google Scholar]
- Yovel G, Levy J, Yovel I. Hemispheric asymmetries for global and local visual perception: effects of stimulus and task factors. J Exp Psychol Hum Percept Perform. 2001;27:1369–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.