Abstract
Background
This study aimed to investigate the growth curve, cell colony formation, cell cycle, apoptosis, anti-anoikis, and ability of invasion, adhesion, and migration of cervical cancer cells after exposure to a model of a simulated CO2 pneumoperitoneum environment with different pressures and at different times.
Material/Methods
The cervical cancer cells were cultured in groups with 8 and 16 mmHg of 100% CO2 for 1, 2, 3, and 4 h in a model of a simulated environment of CO2 pneumoperitoneum. The cells in the control group were cultured in a standard environment. The growth curve was drawn through constant survival cell counts for 7 days, and the group with most obvious change was selected for subsequent experiments to detect cell colony formation, cell cycle apoptosis, and anti-anoikis, and the ability of invasion, adhesion, and migration.
Results
After a brief inhibition, the proliferation of cervical cancer cells was markedly increased and had no relationship with different CO2 pressures. Compared with the control group, the early apoptosis rate in the experimental group was higher, and the ability of invasion, migration, and adhesion decreased significantly.
Conclusions
Cervical cancer cells stimulated by a CO2 pneumoperitoneum environment in vitro have an increased the ability to proliferate after a short period of inhibition and have reduced abilities of invasion, migration, and adhesion.
MeSH Keywords: Apoptosis; Cell Proliferation; Genes, Tumor Suppressor; Pneumoperitoneum, Artificial
Background
In recent years, laparoscopic technique has been widely applied in clinical practice because of its advantages, such as shorter recovery period, less postoperative pain, and better cosmetic effects. However, CO2 pneumoperitoneum is facing some new problems in the treatment of malignancies [1–3], including the dissemination of tumor cells in the area around the incision and the intra-abdominal metastasis of tumor cells, which have become important issues in laparoscopic surgeries [4–6]. There exist various different viewpoints about the effects of CO2 pneumoperitoneum on the growth, apoptosis, and metastasis of cancer cells [7–10]. In this study, we investigated cervical cancer cell growth curve, colony formation, cell cycle, apoptosis, anti-anoikis, and the ability of cell invasion, adhesion, and migration after cervical cancer cells were treated by CO2 in a model of a simulated environment of CO2 pneumoperitoneum. Our aim is to perform a preliminary evaluation of the safety of laparoscopy for the treatment of gynecological malignant tumors and to provide laboratory evidence.
Material and Methods
Cell culture
Cervical HeLa cells (provided by Guangxi Institute of Cancer Prevention and Treatment) were cultured in RPMI-1640 (GIBCO Corporation, USA) culture solution containing 10% fetal bovine serum (FBS) (GIBCO Corporation, USA). The cells were incubated in an environment of 5% CO2 at 37°C, rinsed in phosphate-buffered saline (PBS), and digested by 0.25% trypsin/ethylene diamine tetra-acetic acid (EDTA). The cells in logarithmic phase were used for the follow experiments.
Construction model of CO2 pneumoperitoneum in vitro
We made 2 holes on 2 opposite sides of a Plexiglas container: 1 for intake and 1 for the outlet. The one for air intake is connected with a CO2 pressure bottle through a hose, and the intake gas flow rate and speed were controlled by a CO2 pressure-reducing valve. The outlet was connected to a CO2 pressure measurement device by a hose, which was used to monitor and to control the CO2 pressure at any time. After the glass container was sterilized by 75% ethanol and ultraviolet radiation, the air in the container was drawn out by vacuum pump and the cell culture plates were placed in it. Then the pure CO2 was poured in until the pressure required by the experiment could be achieved and be maintained at certain times. The cell culture plates treated by CO2 were transferred to the normal cell culture incubator for the next culture until the time of the next experiments.
Cell growth curve assay (survival cell count)
The HeLa cells were cultured in groups with 8 and 16 mmHg of 100% CO2 for 1, 2, 3, and 4 h in the model described above. The cells in the control group were cultured in a standard environment. After being treated by CO2, 3 whole cells in each group/per day were picked out for the spectrophotometric assay experiment, which used 3-[4,5Dimethylthiazol-2-yl], 5-diphenyltetrazolium bromide, or thiazolyl blue (MTT) assay. Optical density readings were measured at 450 nm. The growth curve was drawn with the vertical axis for optical density (OD) and the horizontal axis for time. The group with the most obvious change was selected for the following experiments.
Cell colony formation experiment
The HeLa cells were prepared with a single-cell suspension and cultured in 24-well plates according to the gradient density of 50, 100, and 200 cells per dish and were gently shaken by cross direction to disperse cells evenly. The cells were then cultured in the CO2 incubator (5%) at 37°C for 7 days, then the cells were rinsed with PBS, fixed by methanol, and stained by Giemsa in the standard manner. Groups of more than 50 cells counted under the microscope were considered as a clone. The ratio of clone formation was calculated with the following formula:
The experimental procedures were repeated 3 times, and the average of 3 ratios was the final ratio of clone formation.
Determination of cell cycle and proliferative index
The cells were collected 1, 3, 5, and 7 days after CO2 treatment as described above. The cells were digested in EDTA, washed with PBS, and fixed by 70% cold ethanol at 4°C for 48 h. Then the cells were centrifuged and digested with RNase A (50 ug/ml) for 0.5 h and stained with propidium iodide (PI) staining fluid (50 ug/ml)at 4°C for 1 h. Approximately 1~5×106 cells were prepared for the content distribution of DNA detected by flow cytometry (Beckman Corporation, USA). The changes in distribution of cell in the cell cycle were analyzed by Multicycle software. The proliferative index was determined by the percentages of cells in the G1, G2, and S phases and calculated as follows:
Determination of apoptosis and anti-anoikis
The dish treated by poly hydroxyethyl methacrylic acid (Poly-HEMA) was used for cell adhesive culture and suspending culture. The percentage of apoptotic cell of the adhesive culture and suspending culture was calculated by FITC-labeled Annexin V and PI double-staining flow cytometry. The apoptotic index was determined by the DNA distribution of cells just before G1 and after cell debris.
Determination of the ability of cell invasion in vitro
The lower surface of a polycarbonate membrane (8-μm aperture) in the model of Transwell unit (Corning Costar Corporation, USA) was treated by 50 μl PBS containing 5 μg fibronectin (FN) (Biological Center of Beijing University, China), and then was dried overnight at room temperature. We added 60 ul of Matrigel (Sigma Corporation, USA) into 300 ul of serum-free medium, 100 ul of which were added into the upper cabin of the Transwell, and then was incubated in the cell incubator at 37°C for 5 h. The cells were digested and 1×105 cells were added into the upper cabin. After being treated by CO2, the cells in the model of the Transwell unit were incubated for 1, 3, 5, and 7 days respectively in the normal cell incubator. Then 500 ul of conditioned medium containing 20% FBS was added into the lower cabin. The cells were washed by PBS, fixed by 5% glutaraldehyde at 4°C and stained by Giemsa. The cells on the lower surface were counted using a 100-power microscope in 10 views of each membrane after the cells on the upper surface were wiped by the cotton balls. The average of each view was considered as the final result. The experimental procedures were repeated 5 times.
Determination of the ability of cell migration in vitro
The experimental procedures were similar to those used for determining cell invasion ability, except the Matrigel was not added into the upper Transwell cabin.
Determination of the ability of cell adhesion in vitro
We added 50 μl of FN (20 mg/L) into each well of 96-well plates, dried overnight and kept at 4°C. We added 100 μl of 1% albumin from bovine serum (BSA) (Sigma Corporation, USA) in each well for sealing the binding site. The cells were digested and added into each well at 1, 3, 5, and 7 days after CO2 treated. Non-adhered cells were washed off by PBS after 90 min of incubation at 5% CO2 at 37°C, and the adhered cells were measured by the value of A450 nm of MTT assay. The experimental procedures were repeated 5 times.
Statistical analysis
All data were statistically analyzed by SPSS l3.0 software and the data of different groups were analyzed by repeated-measures variance. The results as indicated by χ̄±s and the t-test were used for the group analysis. The difference was considered statistically significant at P<0.05.
Results
Growth curve results
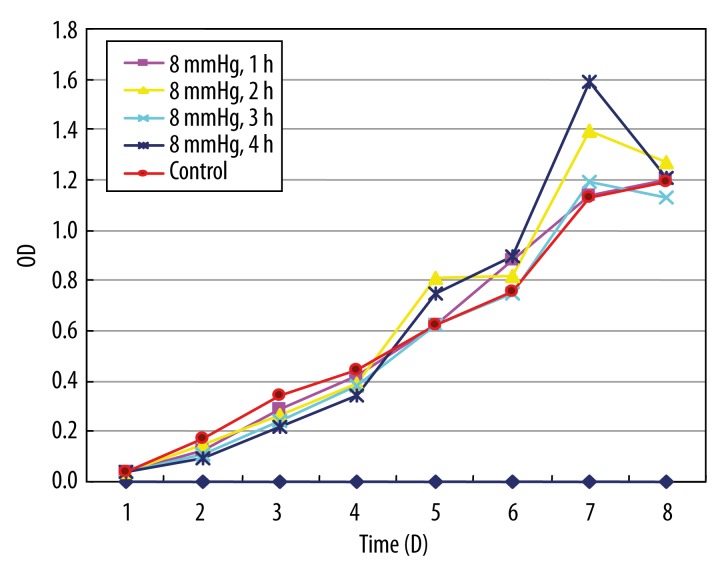
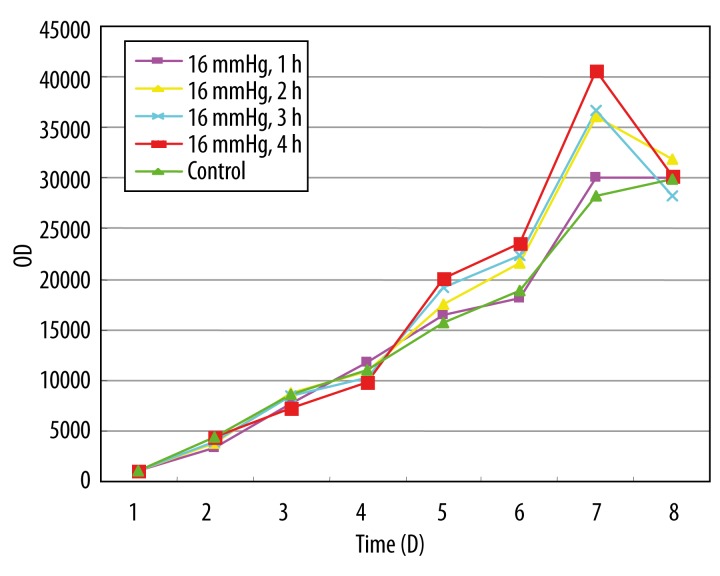
Figures 1 and 2 show the growth curves about the HeLa cells treated with 8 and 16 mmHg of CO2 for 1, 2, 3, and 4 h. In the first 3 days, the rate of growth in the 2 experimental groups was slightly lower than in the control group. From the 4th day, the rate of growth in the experimental groups increased significantly with time, compared with the control group. There was no significant difference between the high-pressure group (16 mmHg) and the low-pressure group (8 mmHg). The high-pressure group was selected for the following experiments.
Figure 1.
The growth curve of cervical cancer cell treated by 8 mmHg CO2 stimulation. The growth curve was drawn with the vertical axis for optical density (OD) and the horizontal axis for time.
Figure 2.
The growth curve of cervical cancer cell by 16 mmHg CO2 stimulation. The growth curve was drawn with the vertical axis for optical density(OD) and the horizontal axis for time.
The results of cell colony formation
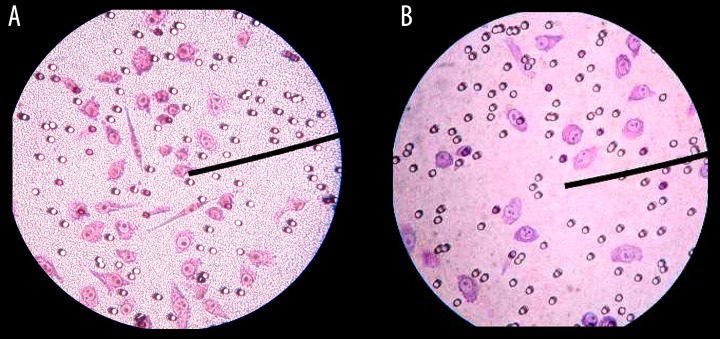
The different levels of cell colony formation were shown on the 7th day after being stimulated by CO2: 25.1±5.4% for the experimental group and 15.6±3.6% for the control group. Compared with the control group, the cell colony formation increased significantly (P<0.05) after being stimulated by CO2 (Figure 3).
Figure 3.
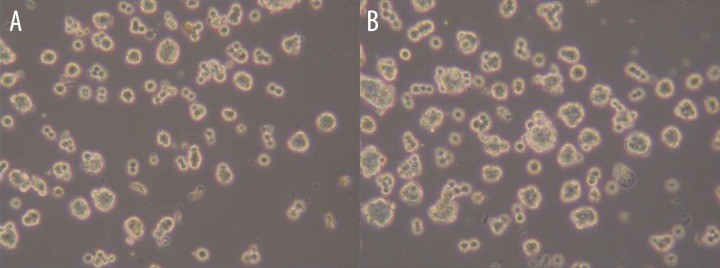
The cell colony formation in the 7th day of the control group and (16 mmHg, 4 h) group. (A) control group in 7th day, (B) CO2 group in 7th day.
Proliferative index results
Compared with the control group, the proliferative index of the HeLa cells tended to be lower at 1 and 3 days after being treated by CO2 (P<0.05), but the proliferation index in the experimental groups increased gradually with the extension of incubation time. After 7 days of CO2 exposure for 4 h, the proliferation index was higher in the experimental group than that of the control group (P<0.05) (Table 1).
Table 1.
The cell proliferative index in the groups (χ̄± s).
| Group | Cell proliferative index | |||
|---|---|---|---|---|
| 1d | 3d | 5d | 7d | |
| Control | 81.4±7.3 | 84.2±9.6 | 79.0±7.8 | 80.5±9.3 |
| 16 mmHg, 4 h | 61.6±8.1* | 66.6±7.2* | 77.8±7.9 | 89.8±8.8* |
Compare with control group P<0.05.
The results of apoptosis and anti-anoikis
On the scatter plot made by a double-variable flow cytometry instrument, the cells in the left lower quadrant were living cells (FITC−/PI−), the cells in the upper right quadrant were dead cells (FITC+/PI+), and the cells in the lower right quadrant were apoptosis cells (FITC+/PI−). The results show that with the extension of incubation time after stimulated by CO2, the cell apoptosis rate of the control group and experimental group both increased (P<0.05). Compared with the control group, the early apoptosis rate in the experimental group was higher at the same time point of both adhesive culture and suspending culture. There was no significant difference in early apoptosis rate between adhesive culture and suspending culture (Table 2).
Table 2.
The comparison of cell AnnexinV+ in different cultivation condition of two groups.
| Group | 1d | 3d | 5d | 7d | ||||
|---|---|---|---|---|---|---|---|---|
| Adhesive culture | Suspending culture | Adhesive culture | Suspending culture | Adhesive culture | Suspending culture | Adhesive culture | Suspending culture | |
| Control | 0.41 | 0.41 | 0.44 | 0.45 | 1.32* | 1.04* | 2.45* | 2.64* |
| 16 mmHg, 4 h | 1.18# | 1.17# | 1.23# | 1.27# | 3.79*,# | 3.93*,# | 4.53*,# | 4.78*,# |
Compare with 1d P<0.05;
compare with control group P<0.05.
The results of the ability of cell invasion, migration, and adhesion in vitro
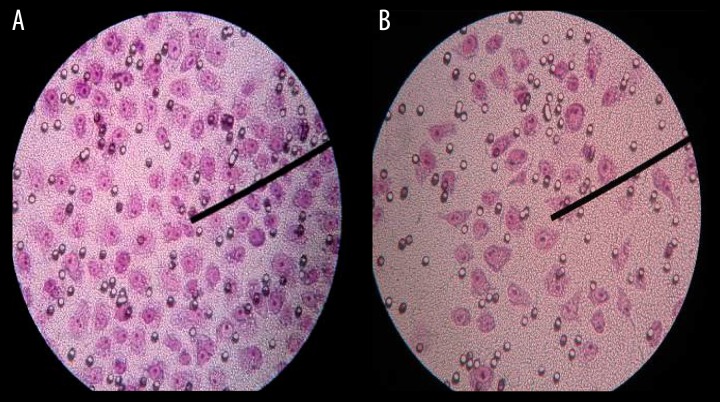
Figures 4 and 5 show that the HeLa cells passed through the membrane surface of the transwell chambers in the cell invasion and migration experiment. Compared with the control group, the ability of cell invasion in the experimental groups was inhibited after treatment with CO2 (P<0.05) (Table 3), the cell migration ability in the experimental group was inhibited at 1, 3, and 5 days after stimulation by CO2 (P<0.05), and there was no significant difference on the 7th day (Table 4). There were significant differences in A450 nm value of MTT between the experimental group and the control group at 1 and 3 days (P<0.05), and there was no significant difference on the 5th and 7th days (Table 5).
Figure 4.
The Hele cells passed through the membrane surface of the transwell chambers in the cell invasion experiment in the 1th day. (A) contorl group, (B) (16 mmHg, 4 h) group.
Figure 5.
The Hele cells passed through the membrane surface of the transwell chambers in the cell migration experiment in the 1th day. (A) control group, (B) (16 mmHg, 4 h) group.
Table 3.
The ability of cell invasion in the groups (χ̄± s).
| Group | The cell count | |||
|---|---|---|---|---|
| 1d | 3d | 5d | 7d | |
| Control | 48.4±4.3 | 87.2±2.6 | 50.0±3.8 | 49.5±4.3 |
| 16 mmHg, 4 h | 19.6±2.1* | 24.6±3.9* | 30.8±5.7* | 37.8±3.8* |
Compare with control group P<0.05.
Table 4.
The ability of cell migration in the groups (χ̄± s).
| Group | The cell count | |||
|---|---|---|---|---|
| 1d | 3d | 5d | 7d | |
| Control | 106.6±12.9 | 101.1±8.2 | 98.8±7.9 | 101.4±8.0 |
| 16 mmHg, 4 h | 75.8±9.4* | 81.0±9.4* | 86.0±8.2* | 96.4±7.6 |
Compare with control group P<0.05.
Table 5.
The adhesive ability of Hele cell in the groups (χ̄± s).
| Group | Optical density (OD) | |||
|---|---|---|---|---|
| 1d | 3d | 5d | 7d | |
| Control | 0.715±0.092 | 0.642±0.057 | 0.674±0.063 | 0.702±0.072 |
| 16 mmHg, 4 h | 0.359±0.052* | 0.466±0.014* | 0.636±0.046 | 0.691±0.065 |
Compare with control group P<0.05.
Discussion
Our study results indicates that the proliferation of cervical cancer cells is markedly enhanced, having no association with the CO2 pressure after a short period of inhibition in the condition of CO2 pneumoperitoneum in vitro. Moreover, the cell colony formation increased significantly after stimulation by CO2. This result is similar that reported by Gutt [11], who cultivated the CX-2 (human rectal adenocarcinoma cell) and DAN-G (human pancreatic cancer cell) at 0, 0.8, and 1.6 kPa of CO2. Also, Smidt et al. [8] found that CO2 exposure increased the growth of human ovarian tumor SKOV3 cells in vitro. However, Jiang [10 ]reported inhibition of the growth of HO8910 and SKOV3 in vitro in the condition of CO2, which was associated with time and with the CO2 pressure. Neuhaus et al. [12] found that CO2 did not increase the growth of DAMA tumor cells.
According to our study results, the mechanism of the change of cell growth after simulation by CO2 may attribute to the changed cell cycle. The change trend of the cell cycle was consistent with the result of the growth curve. The proliferative index determined by the cell cycle result tended to be lower at 1 and 3 days after exposure to CO2 for 4 h, and the proliferation index increased gradually with the extension of incubation time, which was higher in the experimental group in the control group on the 7th day. Currently, it is not clear how CO2 affects cancer cells. Some studies showed that this effect was achieved by the influence of the transition of cells from G1 to S phase [13], and others found that the acidic environment of CO2 could activate mitosis enzymatic activity promoting tumor growth [14].
On the other hand, the Transwell cabin model was applied to determinate the change in ability of cancer cells to invade, metastasize, and adhere, and the results of our experiments indicate that CO2 inhibits the invasive, metastasis, and adhesive ability of cervical cancer cells after exposure to the simulated CO2 pneumoperitoneum environment in vitro. Results of research on the metastasis ability of cancer cells stimulated by CO2 are not consistent. Our results are similar to the report of Ma et al. [9], which indicated that CO2-insufflation induced a temporary change in the adhesion and invasion capacity of cancer cells in vitro, and reported that higher CO2 insufflation pressure inhibited adhesion, invasion, and metastatic potential. Kim et al. [15] studied the effect of the CO2 pneumoperitoneum model in vitro on the expression of E-cad, CD4, ICAM-1 (intracellular adhesion molecule 1), and ICAM-2 (intracellular adhesion molecule 2), and the result indicated that CO2 had a temporal effect on the expression of adhesion molecules on the surface of cancer cells in vitro. FengBo et al. proved that with the increase of CO2 pneumoperitoneum pressure, the expression of E-cad, CD4, and ICAM-1 decreased gradually. The study of Yang et al. [16] indicated that high pressure of CO2 pneumoperitoneum played an important role in suppressing the expression of the chemokine receptors CXCR4 and CCR7 in colorectal carcinoma cells in vitro.
In our study, both the experiment group and control group cells had apoptosis and anti-anoikis abilities, but the anti-anoikis of experiment group was significantly worse than that of the control group. This result indicates that CO2 inhibits the anti-anoikis ability, which may be one of the reasons for the decreased invasive and metastasis ability of cervical cancer cells [17,18]. We also think that the gradual recover of the ability of the invasion, migration, and adhesion in cervical cancer cells was due to the changes in culture medium and transfer of culture.
Through this experiment, we showed that cell stimulation by CO2 is not the main reason for the dissemination of tumor cells intra-abdominally and around the incision. Our results suggest that the ability of malignancy to spread in laparoscopic surgeries is mainly due to the direct implantation of cancer cells at trocar sites during extraction of the tumor specimen, with spilling of exfoliated cells, and excessive and non-standard manipulation of the tumor.
Conclusions
Cervical cancer cells stimulated by the CO2 pneumoperitoneum environment in vitro can increase the ability of proliferation after a short time of inhibition and reduce the ability of invasion, migration, and adhesion. Further in vivo studies are needed.
Footnotes
Conflict of interest statement
All the authors have no conflicts of interest to declare.
Source of support: The following grants and foundations supported this work: Research grant No. 0575088 from the Natural Science Foundation of Guangxi Province of China
References
- 1.Lehner M, Wenzl R, Heinzl H, et al. Influence of delayed staging laparotomy after laparoscopic removal of ovarian masses later found malignant. Obstet Gynecol. 1998;92:967–71. doi: 10.1016/s0029-7844(98)00323-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen TP, Liu HP, Lu HI, et al. Incidence of incisional recurrence after thoracoscopy. Surg Endosc. 2004;18(3):540–42. doi: 10.1007/s00464-003-8215-9. [DOI] [PubMed] [Google Scholar]
- 3.Daniels IR. Port-site tumour recurrence of oral squamous carcinoma following percutaneous endoscopic gastrostomy: a lesson to be aware of. World J Surg Oncol. 2006;19(4):64. doi: 10.1186/1477-7819-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson H, Sargent D, Wieand HS, et al. Clinical Outcomes of Surgical Therapy Study Group: A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–59. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 5.Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 6.Zheng MH, Feng B, Lu AG, et al. Laparoscopic versus open right hemicolectomy with curative intent for colon carcinoma. World J Gastroenterol. 2005;11:323–26. doi: 10.3748/wjg.v11.i3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecuru F, Agostini A, Camatte S, et al. Impact of pneumoperitoneum on tumor growth: results of an experiment on two ovarian carcinoma models. Surg Endosc. 2002;16:1170–74. doi: 10.1007/s00464-001-9226-z. [DOI] [PubMed] [Google Scholar]
- 8.Smidt VJ, Singh DM, Hurteau JA, Hurd WW. Effect of carbon dioxide on human ovarian carcinoma cell growth. Am J Obstet Gynecol. 2001;185:1314–17. doi: 10.1067/mob.2001.119079. [DOI] [PubMed] [Google Scholar]
- 9.Ma JJ, Feng B, Zhang Y, et al. Higher CO2-insufflation pressure inhibits the expression of adhesion molecules and the invasion potential of colon cancer cells. World J Gastroenterol. 2009;15(22):2714–22. doi: 10.3748/wjg.15.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng J, Lang J, Jiang Y, et al. Impact of different pressures and exposure times of a simulated carbon dioxide pneumoperitoneum environment on proliferation and apoptosis of human ovarian cancer cell lines. Surg Endosc. 2006;20:1556–59. doi: 10.1007/s00464-005-0560-4. [DOI] [PubMed] [Google Scholar]
- 11.Gutt CN, Kim ZG, Hollander D, et al. CO2 environment influences the growth of cultured human cancer cells dependent on insufflation pressure. Surg Endosc. 2001;15(3):314–18. doi: 10.1007/s004640000321. [DOI] [PubMed] [Google Scholar]
- 12.Neuhaus SJ, Ellis TS, Barrett MW, et al. In vitro inhibition of tumor growth in a heliumrich environment: implication for laparoscopic surgery. Aust N Z J Surg. 1999;69:52–55. doi: 10.1046/j.1440-1622.1999.01495.x. [DOI] [PubMed] [Google Scholar]
- 13.Gu Z, Ding G, Liang K, et al. TESTIN suppresses tumor growth and invasion via manipulating cell cycle progression in endometrial carcinoma. Med Sci Monit. 2014;14(20):980–87. doi: 10.12659/MSM.890544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Hirabayashi Y, Shiromizu A, et al. Enhancement of port site metastasis by hyaluronie acid under CO2 pneumoperitoneum in a murine model. Surg Endosc. 2001;15(5):504–7. doi: 10.1007/s004640090016. [DOI] [PubMed] [Google Scholar]
- 15.Kim ZG, Mehl C, Lorenz M, Gutt CN. Impact of Laparoscopic CO2-insufflation on Cancer-associated Molecules in Cultured Colorectal Cancer Cells. Surg Endosc. 2002;16(8):1182–86. doi: 10.1007/s00464-001-9194-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang CK, Li GD, Ying MG, Xu K. Effect of CO2 pneumoperitoneum on the expression of the chemokine receptors CXCR4 and CCR7 in colorectalcarcinoma cells in vitro. Chin Med J (Engl) 2013;126(24):4747–51. [PubMed] [Google Scholar]
- 17.Qiu JX, He YQ, Wang Y, et al. Plumbagin induces the apoptosis of human tongue carcinoma cells through the mitochondria-mediated pathway. Med Sci Monit Basic Res. 2013;19:228–36. doi: 10.12659/MSMBR.884004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tvrdík D, Skálová H, Dundr P, et al. Apoptosis-associated genes and their role in predicting responses to neoadjuvant breast cancer treatment. Med Sci Monit. 2012;18(1):BR60–67. doi: 10.12659/MSM.882205. [DOI] [PMC free article] [PubMed] [Google Scholar]