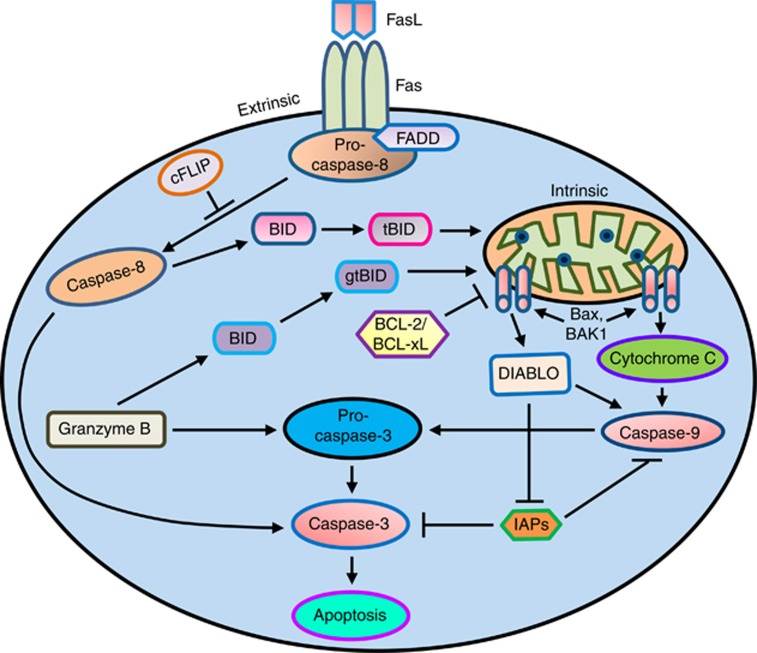
Figure 1.
Apoptosis initiation by caspases: apoptosis can be initiated by the two major pathways involving initiator and effector caspases. The death-receptor (extrinsic) pathway acts through caspase-8 while the mitochondrial (intrinsic) pathway involves caspase-9. Both pathways converge to activate the effector caspase-3, which act on the death substrates. Caspase-8 can cleave the BH3-only protein Bcl-2 interacting domain death agonist (BID), forming a truncated BID (tBID), which can activate the intrinsic apoptotic pathway. Granzyme B is a cytotoxic cell proteinase-1, which directly cleaves and activates pro-caspase-3. Granzyme B can also cleave BID, resulting in granzyme tBID, which can activate the intrinsic apoptotic pathway. In addition to the caspases, cell death is regulated by Bcl-2 and inhibitor of apoptosis (IAP) protein families. Bcl-2, Bcl-XL proteins are thought to regulate the mitochondrial permeability transition, thereby inhibiting cytochrome c release, while BAX and Bcl-2-antagonist/killer 1 (BAK-1) promote cytochrome c release, causing caspase-9 activation, which then leads to the activation of caspase-3 and promotes apoptosis. The IAP proteins act downstream to prevent processing of initiator caspase-9, and inhibiting the activity of the effector caspase-3. Pro-apoptotic mitochondrial factor, DIABLO (direct IAP protein-binding protein with low pI; also known as SMAC) is released and contributes to apoptosis by activating caspase-9 or inhibiting IAPs. cFLIP (cellular FLIP/caspase-8 inhibitor protein) acts as a negative regulator of the activation of caspase-8