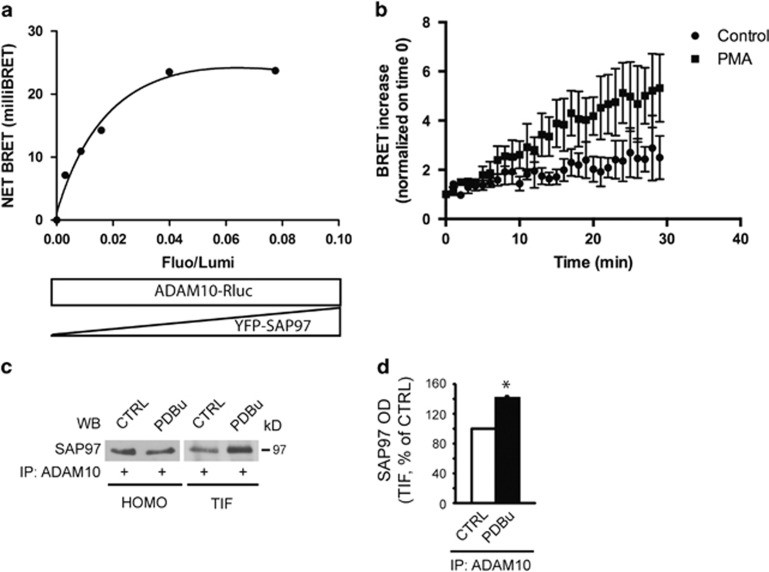
Figure 1.
PKC activation promotes ADAM10/SAP97 association. (a) BRET experiments in living human embryonic kidney 293 (HEK293) cells. We fused the C terminus of ADAM10 to the energy donor Renilla luciferase (ADAM10-Rluc) and the N terminus of SAP97 to the acceptor YFP (YFP-SAP97). Under the condition of a constant level of ADAM10-Rluc expression, BRET signal increased hyperbolically as a function of YFP-SAP97 expression level. Saturation of the BRET signal when all the donor molecules were linked to the acceptor indicated a specific interaction between ADAM10 and SAP97 proteins (R2=0.9824, nonlinear regression equation, assuming a single binding site; GraphPad Prism, La Jolla, CA, USA). (b) Phorbol 12-myristate 13-acetate (PMA) treatment increases BRET signal after 30 min in cells transfected with ADAM10-Rluc and YFP-SAP97 (Fluo/Lumi=0.091±0.009). Data represent mean±S.E.M. of three independent experiments. (c) Total homogenates (HOMO) and TIF of control (CTRL) and PDBu-treated hippocampal slices were immunoprecipitated (IP) with a pAb to ADAM10 and SAP97 co-precipitation was evaluated. PDBu increases ADAM10/SAP97 co-precipitation only in TIF but not in HOMO. (d) Quantification of experiments in (c) (n=3, * P=0.006, PDBu versus CTRL, paired t-test). In this and all subsequent figures, data represent mean±S.E.M.