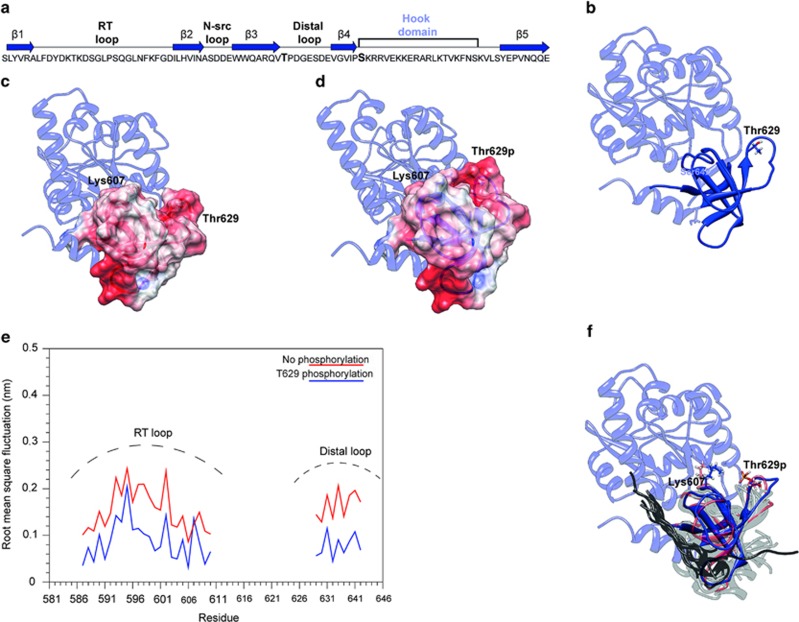
Figure 4.
PKC phosphorylation of the T629 residue affects SAP97 structure. (a) Aa sequence and topology of the SH3 domain of SAP97. The blue arrows represent the β-strands and the lines represent the loops. The HOOK domain is also indicated. Residues T629 and S642 are shown in bold. (b) Ribbon representation of the SH3-HOOK-GK domains of SAP97. The SH3 domain is shown in blue and the HOOK and GK domains in light blue. Residues T629 and S642 are shown as blue sticks. (c and d) Electrostatic potential mapped on the molecular surface of the SH3 domain in the absence (c) or presence (d) of the phosphorylation on residue T629. The HOOK and GK domains are shown in light blue, whereas the SH3 domain is colored according to the electrostatic potential, blue is positive, white is neutral and red is negative. Residues K607 and T629 are shown as sticks. (e) The RMSF of the RT and distal loops. Per-residue RMSF of the RT and distal loops in the presence (blue line) and in the absence (red line) of the T629 phosphorylation. (f) Ribbon representation of the SH3 domain of SAP97 extracted from the simulation in the absence (red) or presence (blue) of the phosphorylation. The remaining of the protein is in light blue. The SH3 domains superimposed with the SAP97-SH3 domain are shown in light grey, and the cocrystallized peptides in dark grey. Residues T629 and K607 are shown as sticks