Scheme 2. Preparation of Analogue Precursorsa.
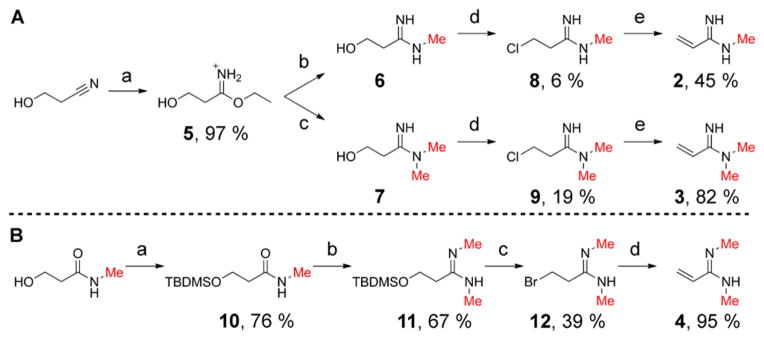
a(A) Synthesis of monomethyl and asymmetric dimethyl analogue precursors: (a) HCl, EtOH, Et2O, 3 h; (b) MeNH2, MeOH, 19 h; (c) Me2NH, MeOH, 16 h; (d) PPh3, CCl4, DMF, 22–24 h; (e) triethylamine, acetonitrile, 1–5 min. (B) Synthesis of symmetric dimethyl analogue precursor (a) TBDMSCl, imidazole, DMF, 24 h; (b) i. Et3OBF4, DCM, 1 h; ii. MeNH2, DCM/THF, 1 h; (c) PPh3Br2, DCM, 20 h; (d) triethylamine, acetonitrile, 1 min. All reactions were performed at room temperature.
