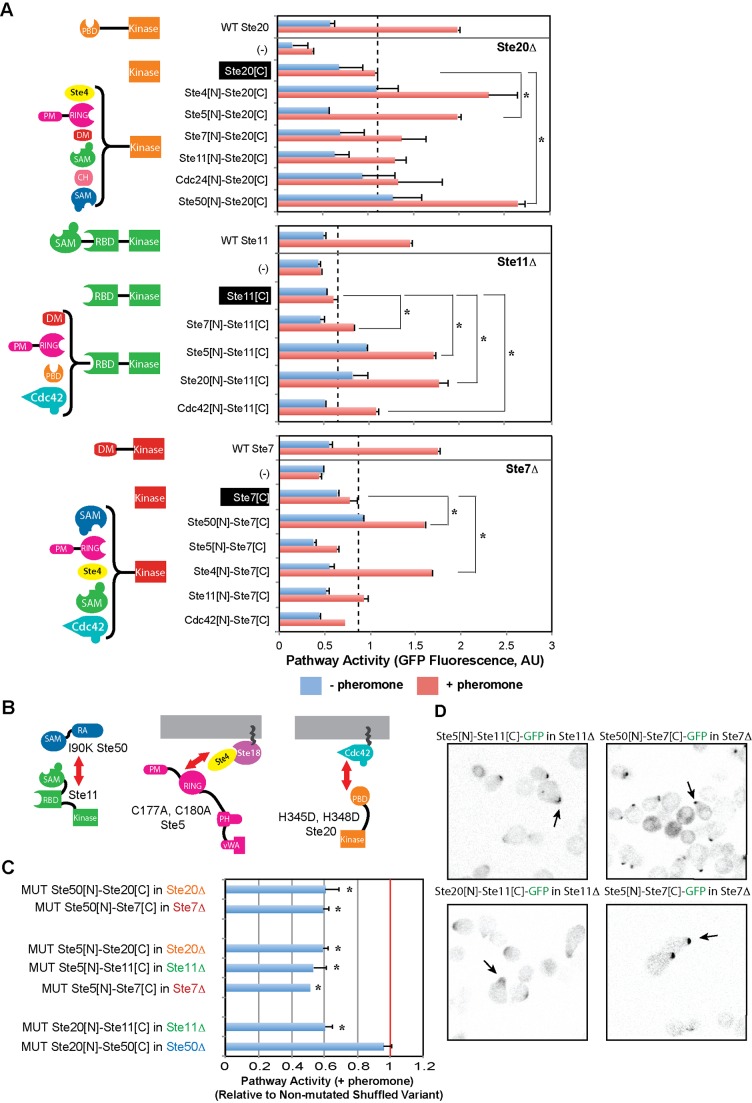
Figure 3. Kinases are recruited to the signaling complex by alternative N-t localization domains.
(A) Comparison of mating pathway activation by kinase variants with or without N-t localization domains. In all cases, deletion of the full length kinase gene results in pathway inactivation. Expression of kinase variants lacking N-t localization domains only recovers partial pathway activation. In contrast, rearrangement events that fuse N-t domains known to interact with diverse partners in the mating signaling complex to the C-t kinase domains restore pathway activation to higher levels. (B) Schematic representation of the mutations introduced in Ste50 SAM domain, Ste5 RING domain, and Ste20 PBD domain. (C) Pathway activity for domain rearrangement variants carrying the mutations shown in (B) relative to the activity of the corresponding non-mutated variants. In most cases, mutations that disrupt specific recruitment interactions decrease pathway activation between 40%–50%. MUT Ste20[N]-Ste50[C] might still localize to the signaling complex, as Ste50's RA domain binds Cdc42 independently of Ste20's PBD domain [53]. (D) Fluorescence microscopy of GFP-tagged domain rearrangement variants shows that kinases can be recruited to the mating shmoo using alternative interaction domains. Statistically significant differences are marked with asterisks. Data shown in Data S1.