Abstract
Polar auxin transport (PAT) plays key roles in the regulation of plant growth and development. Flavonoids have been implicated in the inhibition of PAT. However, the active flavonoid derivative(s) involved in this process in vivo has not yet been identified. Here, we provide evidence that a specific flavonol bis-glycoside is correlated with shorter plant stature and reduced PAT.
Specific flavonoid-biosynthetic or flavonoid-glycosylating steps were genetically blocked in Arabidopsis thaliana. The differential flavonol patterns established were analyzed by high-performance liquid chromatography (HPLC) and related to altered plant stature. PAT was monitored in stem segments using a radioactive [3H]-indole-3-acetic acid tracer.
The flavonoid 3-O-glucosyltransferase mutant ugt78d2 exhibited a dwarf stature in addition to its altered flavonol glycoside pattern. This was accompanied by reduced PAT in ugt78d2 shoots. The ugt78d2-dependent growth defects were flavonoid dependent, as they were rescued by genetic blocking of flavonoid biosynthesis. Phenotypic and metabolic analyses of a series of mutants defective at various steps of flavonoid formation narrowed down the potentially active moiety to kaempferol 3-O-rhamnoside-7-O-rhamnoside. Moreover, the level of this compound was negatively correlated with basipetal auxin transport.
These results indicate that kaempferol 3-O-rhamnoside-7-O-rhamnoside acts as an endogenous PAT inhibitor in Arabidopsis shoots.
Keywords: Arabidopsis thaliana, flavonol biosynthesis, flavonol glycoside, flavonol glycosyltransferases, plant growth, polar auxin transport
Introduction
The phytohormone auxin, represented predominantly by indole-3-acetic acid (IAA), plays a crucial role in plant growth and development. Auxin needs to be transported from the sites of synthesis, mainly in the apices and young leaves, to the distal part of the plant to exert its function (Berleth et al., 2007). Auxin transporters, including ABCB proteins, AUX1/LAX family members and PIN proteins (Noh et al., 2001; Friml, 2003; Petrasek et al., 2006; Yang et al., 2006; Zažímalová et al., 2010; Peer et al., 2011), are responsible for the auxin fluxes and patterning in plants (Friml et al., 2003). Flavonoids, phenylpropanoic secondary metabolites, have been implicated in the blocking of auxin transport (Peer & Murphy, 2007). The supply of flavonols to detached zucchini hypocotyls resulted in decreased polar auxin transport (PAT) (Jacobs & Rubery, 1988). In Arabidopsis, PAT was increased in transparent testa4 (tt4), a flavonoid-deficient mutant defective in the first step of flavonoid production (Shirley et al., 1995; Buer & Muday, 2004; Peer et al., 2004) (Fig.1). Accordingly, tt4 roots exhibited delayed gravitropism, which was reversed by chemical complementation by naringenin, an intermediate of flavonoid biosynthesis (Buer & Muday, 2004). By contrast, PAT was reduced in the flavonol over-production mutant tt3 defective in dihydroflavonol reductase (Fig.1), consistent with an inhibitory role of flavonols in PAT (Peer et al., 2004). Despite these and other substantial pieces of evidence supporting a role of flavonols in the modulation of auxin transport (Kuhn et al., 2011; Lewis et al., 2011; Grunewald et al., 2012), neither specific flavonol aglycones nor their conjugates active in this process in vivo have been identified so far.
Figure 1.
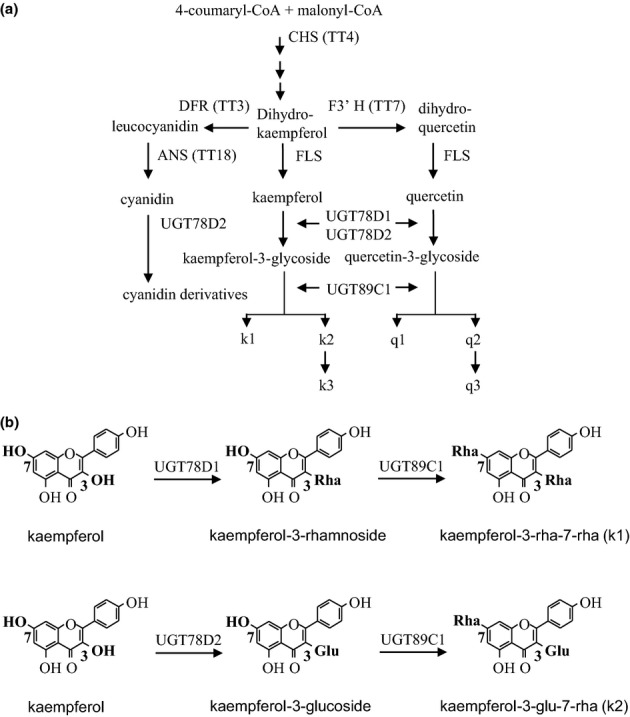
Flavonoid biosynthesis pathway in Arabidopsis thaliana. (a) Scheme of flavonoid biosynthesis. CHS (TT4), chalcone synthase; F3′H (TT7), flavonoid 3′-hydroxylase; DFR (TT3), dihydroflavonol 4-reductase; FLS, flavonol synthase; ANS (TT18), anthocyanidin synthase; UGT78D1, flavonol 3-O-rhamnosyltransferase; UGT78D2, flavonoid 3-O-glucosyltransferase; UGT89C1, flavonol 7-O-rhamnosyltransferase. As shown by Yin et al. (2012), the combined loss of UGT78D1 and UGT78D2 does not imply an accumulation of flavonol aglycones because of a feedback inhibition of flavonol biosynthesis. (b) Glycosylation reactions catalyzed by UGT78D1, UGT78D2 and UGT89C1 (Jones et al., 2003; Tohge et al., 2005; Yonekura-Sakakibara et al., 2007). Abbreviations: Kaempferol (k); rhamnoside (rha); glucoside (glu). k1, k-3-O-rha-7-O-rha; k2, k-3-O-glu-7-O-rha; k3, k-3-O-[rha (1->2 glu)]-7-O-rha; q1, q2 and q3 are quercetins structurally equivalent to k1, k2 and k3, respectively.
The difficulty in relating specific flavonols to auxin transport modulation is, in part, a result of the complex flavonol modification in planta. Flavonol aglycones are intensively modified by UDP-dependent glycosyltransferases (UGTs), which include UGT78D1, UGT78D2, UGT78D3, UGT73C6 and UGT89C1 in the model plant Arabidopsis thaliana (Fig.1) (Jones et al., 2003; Tohge et al., 2005; Yonekura-Sakakibara et al., 2007, 2008). The glycosides are distributed in an organ-specific manner. In contrast with the complex flavonol profile in flowers, it is rather simple in inflorescence stems (Yonekura-Sakakibara et al., 2008; Stracke et al., 2010b). Thus, the inflorescence stem, which is implicated in basipetal auxin movement, is the optimal organ for searching for the flavonol derivative(s) active in auxin transport modulation.
Here, we show that the loss of the flavonoid 3-O-glucosyltransferase UGT78D2 resulted in an altered flavonol glycoside pattern and reduced PAT in shoots, which was accompanied by a reduced plant height and increased branching. Blocking of flavonoid biosynthesis and/or glycosylation at specific steps clearly related the enhanced accumulation of kaempferol 3-O-rhamnoside-7-O-rhamnoside (k1) to the growth defects of ugt78d2. Through analyses of auxin transport in several genotypes, which contained different levels of k1, an inverse correlation between basipetal auxin transport and k1 level was identified. Therefore, we propose that k1 acts as an endogenous auxin transport inhibitor in Arabidopsis shoots.
Materials and Methods
Plant material and growth conditions
The ugt78d1 (SAIL_568_F08), ugt78d2 (SALK_049338), ugt89c1 (SALK_071113), tt4 (SALK_020583), tt7 (GK_349F05) and tt18 (SALK_028793) mutants are based on Arabidopsis thaliana (L.) Heynh., accession Columbia (Col-0). These mutant lines have been described in previous studies (Abrahams et al., 2003; Alonso et al., 2003; Jones et al., 2003; Rosso et al., 2003; Tohge et al., 2005; Yonekura-Sakakibara et al., 2007; Stracke et al., 2010b). The tt3-1 (NASC_N84; Ler background) and fls1 (Riken_PST16145; Nö background) mutants have different genetic backgrounds (Shirley et al., 1995; Ito et al., 2005).
tt4 ugt78d2, tt7 ugt78d2, ugt78d1 ugt78d2, ugt89c1 ugt78d2, tt18 ugt78d2 and fls1 ugt78d2 double mutants were generated by genetic crossing and confirmed by PCR genotyping. Plants were grown in a sun simulator chamber, which provides a photobiological environment very close to natural global solar radiation (Seckmeyer & Payer, 1993; Thiel et al., 1996). Plants were exposed to photosynthetically active radiation (PAR, 400–700 nm) of 200–230 μmol m−2 s−1 with a 16 h : 8 h, light : dark regime at a temperature of 22°C and a relative humidity of 70%. Similar phenotypes were observed when grown in a glasshouse with higher natural light intensities at temperatures of 20–25°C (European springtime).
A DR5::β-glucuronidase (DR5::GUS) line (Col background; Sabatini et al., 1999) was introgressed into ugt78d2; plants were grown on half-strength Murashige and Skoog (MS) medium agar plates supplemented with 1.5% sucrose for 9 d and stained for β-glucuronidase expression for 1 h at 37°C (Deruère et al., 1999).
Genetic complementation of ugt78d2
A genomic fragment containing the UGT78D2 gene was amplified from Col-0 genomic DNA using the primers 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTCGGTCCAAAGGATTTCAG-3′ and 5′-GGACCACTTTGTACAAGAAAGCTGGGTAGATTTTCTGAGCCGTGCAT-3′. The PCR product was cloned into vector pDONR221 using GATEWAY™ (Invitrogen, Karlsruhe, Germany) recombination, confirmed by DNA sequencing and further recombined into pBGW (Karimi et al., 2002). The resulting binary vector was used to transform ugt78d2 knockout mutant plants by the floral dip method (Clough & Bent, 1998). Transgenic plants with a single insertion of the transgene were selected by segregation analysis for experiments.
Analyses of flavonols
For flavonol extraction, the lower 2.5-cm stem segment of 4-wk-old plants was harvested and immediately frozen in liquid nitrogen. The plant material was thoroughly ground in liquid nitrogen using a mortar and pestle, and further homogenized after the addition of 1 ml of methanol per 100 mg of fresh material in a Douncer on ice. The suspension was incubated for 1 h with moderate rotation at 4°C. The extracts were then clarified by centrifugation at 14 000 g for 10 min with a table-top centrifuge at 4°C. One-third volume of distilled water was added to the supernatant, vortexed and centrifuged as above. The cleared extract was analyzed using a reverse-phase high-performance liquid chromatography (HPLC) system (Gemini C18 Phenyl, 5 μm, 150 × 4.6 mm; Phenomenex, Aschaffenburg, Germany). Solvent A was 1% acetic acid and solvent B consisted of 89% methanol with 1% acetic acid. The elution gradient program followed a linear gradient from 20% solvent B to 100% solvent B (75 min) at room temperature. The quantification of k1 was referred to a kaempferol aglycone authentic standard (Carl Roth, Karlsruhe, Germany).
Inflorescence stem gravitropic response assay
Arabidopsis plants were grown on commercial soil substrate (Einheitserde; Einheitserde- und Humuswerke, Sinntal-Altengronau, Germany) for 4 wk in a phytochamber (light intensity, 120 μmol m−2 s–1; 16 h : 8 h, light : dark cycle; relative humidity, 60%; 23°C). Plants were adapted in darkness for 2 h before gravitropic stimulation. Gravitropic stimulation was begun by turning plants by 90° at 23°C in darkness. Except for the 5 min needed for the measurement of the bending angles at each time point, the whole gravitropic response assay was conducted in darkness.
Examination of parenchyma cell size of inflorescence stems
Seven-week-old inflorescence stems were fixed in ethanol : glycerol : water (36 : 1 : 10, v/v/v), hand sectioned along the longitudinal axis, stained with astral blue and safranin, and examined under a microscope in bright field mode. Cell length was measured using Cell^P Professional Imaging Software (Olympus Europe, Hamburg, Germany).
PAT assay
The auxin transport assays were performed as described previously with slight modifications (Okada et al., 1991). Segments (length, 2.5 cm) from the basal part of the primary inflorescence stem were inserted into 30 μl of 1 × MS medium (pH 5.5) with 10.5 μM [3H]-IAA (460 kBq ml−1). Both basipetal and acropetal auxin transport were assayed. One end of the stem fragment (apical end for basipetal auxin transport and basal end for acropetal auxin transport) was dipped into the assay mixture. After 6 h of incubation at room temperature in the dark in a closed vial, a 5-mm segment was cut off from the non-submerged end and measured by scintillation counting. For the inhibition assay, 10 μM of N-1-naphthylphthalamic acid (NPA) was added. The measurements without NPA treatment were compared between each genotype by a paired t-test.
IAA quantification
Col-0 and ugt78d2 plants were grown for 28 d before the inflorescence stems were harvested. The whole inflorescence stem was cut into three segments of equal length, namely the apex, middle and basal segment. The extraction and quantification of free IAA were carried out as described previously, with the endogenous IAA determined by isotope dilution followed by liquid chromatography-mass spectrometry (Dobrev et al., 2005; Dobrev & Vankova, 2012).
Results
Shoot architecture of ugt78d2 plants is altered
A loss-of-function mutant of the flavonoid 3-O-glucosyltransferase UGT78D2 showed a pronounced shoot phenotype (Fig.2). ugt78d2 mutant plants exhibited a significantly shorter stature soon after bolting (Fig.2). At a later growth stage, the mutants developed more branches with an obvious loss of apical dominance (Supporting Information Table S1). To elucidate the mechanism underlying this dwarfism, the cellular architectures of inflorescence stems were examined. In ugt78d2, the parenchymal cell length was reduced significantly when compared with the wild-type. In contrast with stems, other organs were not obviously affected. The lengths of hypocotyls and siliques of ugt78d2 were similar to those of the wild-type (Table S1). Young seedlings and root systems developed in the same way in both genotypes (Fig. S1) Transformation of ugt78d2 with a genomic fragment covering the complete UGT78D2 gene rescued plants from growth disturbances and restored the wild-type phenotype (Fig. S2). Thus, the lesion in UGT78D2 was responsible for the observed shoot growth defects.
Figure 2.

Phenotypes of flavonoid biosynthesis-related mutants. Shoots of Arabidopsis thaliana wild-type and the indicated mutants were imaged after 28 d of growth. Bar, 5 cm.
The ugt78d2 growth phenotype is flavonol dependent
Flavonoids are probable candidates responsible for the ugt78d2 phenotype, as UGT78D2 has been characterized as a flavonoid 3-O-glucosyltransferase and the ugt78d2 mutant exhibits an altered flavonoid pattern (Lee et al., 2005; Tohge et al., 2005). Moreover, transformation of ugt78d2 with the UGT78D2 gene did not only re-establish the wild-type growth phenotype, but also restored its flavonol pattern (Fig.3a). A flavonoid-deficient tt4 ugt78d2 double mutant was generated to further check this effect. The growth defects of ugt78d2 were indeed eliminated in tt4 ugt78d2, indicating that they were flavonoid dependent. Moreover, this observation in the flavonoid-deficient background indicated that the growth defects were not related to the repression of specific flavonol-3-O-glucoside derivatives (k2, k3, q2 and q3) in ugt78d2 (Figs2, 3a). In addition to flavonols, UGT78D2 also recognizes anthocyanidins as substrates (Lee et al., 2005; Tohge et al., 2005). To test the possible involvement of anthocyanins in the development of the ugt78d2 growth phenotype, an anthocyanin-deficient tt18 ugt78d2 double mutant was generated. However, loss of the anthocyanidin synthase in the tt18 ugt78d2 line did not rescue the growth defects of ugt78d2 (Figs2). The flavonol glycoside pattern of tt18 ugt78d2 was similar to that of ugt78d2 (Fig.3a). By contrast, the introgression of fls1 (accession Nö) into ugt78d2, which specifically blocks the biosynthesis of the flavonol moieties, restored wild-type-like growth (Fig. S3). The impact of flavonols was further supported by the slightly repressed growth of the tt3 mutant in comparison with its genetic background Ler; the loss of dihydroflavonol reductase in the tt3 mutant leads to enhanced k1 levels (Fig. S4; Peer et al., 2001). Collectively, these observations indicated that the ugt78d2 growth phenotype was flavonol dependent and that anthocyanins were not responsible for the ugt78d2 growth defects.
Figure 3.
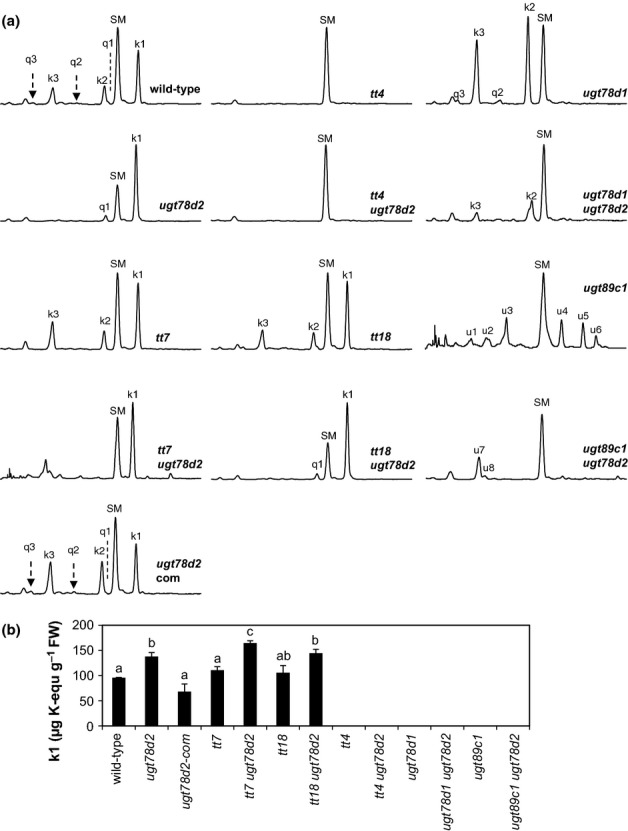
Flavonol glycoside pattern in Arabidopsis thaliana mutants affecting flavonoid biosynthesis and conjugation. (a) Flavonols extracted from stems (lower region of 2.5 cm) of the wild-type and flavonoid metabolism-related mutants. The scale of all representative high-performance liquid chromatograms is identical. Peaks u1–u8 represent unknown flavonoids. SM, sinapoyl malate. (b) Quantification of k1 (Fig.1b) in the wild-type and flavonoid metabolism-related mutants. The means of k1 ± SD from four biological samples (a pool of three to five individual stem segments for one sample) are shown. k1 was quantified using kaempferol aglycone as a standard. Letters above the error bars represent a significant difference between genotypes by a paired t-test (P < 0.01).
The ugt78d2 phenotype is correlated with kaempferol 3-O-rhamnoside-7-O-rhamnoside
To identify the flavonols responsible for the ugt78d2 phenotype, a genetic approach using mutants defective in flavonol biosynthetic and/or conjugating steps was employed. In concert with published data (Yonekura-Sakakibara et al., 2008; Stracke et al., 2010b), six flavonol glycosides were detected in wild-type stems. Three kaempferol glycosides (k1–k3) were the most abundant flavonols, whereas three quercetin glycosides (q1–q3) were present at much lower levels (Fig.3a). No flavonol aglycones were detected in the wild-type and ugt78d2, which is in accordance with previous analyses of the single 3-O-glycosylation mutants ugt78d1 and ugt78d2 (Tohge et al., 2007; Yin et al., 2012) (Fig.1). Furthermore, even the double mutant ugt78d1 ugt78d2, which blocks the major enzymes performing the initial 3-O-glycosylation of the flavonol moiety, did not accumulate aglycones (Yin et al., 2012). In accordance with the loss of 3-O-glucosyltransferase activity in ugt78d2, the 3-O-glucoside derivatives (k2, k3, q2 and q3) were strongly reduced, whereas the 3-O-rhamnoside derivatives (k1 and q1) were elevated, when compared with the wild-type counterpart (Fig.3a). Therefore, both k1 and q1 were positively correlated with the ugt78d2 growth defects.
A tt7 ugt78d2 double mutant was generated in order to further distinguish whether the increases in k1 and/or q1 levels were related to the ugt78d2 growth phenotype. The conversion of kaempferol to quercetin is blocked in tt7 (Fig.1). As expected, tt7 ugt78d2 was devoid of q1 and accompanied by an even larger amount of k1 than in ugt78d2 (Fig.3b). tt7 ugt78d2 was more severely dwarfed than ugt78d2, indicating that a further enhanced k1 level led to a more pronounced phenotype (Fig.2). These observations suggested that the development of the ugt78d2 growth defects was independent of quercetin derivatives, and that k1 was sufficient to induce the ugt78d2 phenotype.
k1 contains 3-O-rhamnosyl and 7-O-rhamnosyl residues (Fig.1b). To further substantiate that k1 was responsible for the observed ugt78d2 phenotype, additional mutants were employed to selectively inhibit the attachment of 3-O- or 7-O-rhamnosyl residues to the k1 backbone. The attachment of these residues to the k1 backbone is catalyzed by UGT78D1 and UGT89C1, respectively (Jones et al., 2003; Yonekura-Sakakibara et al., 2007). As expected, k1 could not be detected in ugt78d2 ugt89c1, but unknown flavonol derivatives accumulated (Fig.3a). Importantly, the ugt78d2 phenotype reverted to a wild-type stature in this double mutant (Fig.2). Likewise, no ugt78d2-like phenotype was observed in ugt78d1 ugt78d2, which was also devoid of k1 (Figs2, 3a). All of these observations clearly indicated that k1 was indeed the flavonol metabolite inducing the ugt78d2 phenotype.
Basipetal auxin transport is reduced in ugt78d2 plants
The dwarf phenotype of ugt78d2 resembled that of the abcb1 abcb19 double mutant, which was greatly impaired in auxin transport (Noh et al., 2001). As flavonols have been postulated to be endogenous auxin transport inhibitors, we reasoned that the increase in k1 suppressed auxin transport in inflorescence stems, resulting in the growth defects. Therefore, polar transport of [3H]-IAA was measured in inflorescence stem segments. NPA-sensitive, basipetal auxin transport in ugt78d2 was reduced significantly to c. 45% of the wild-type level, whereas acropetal auxin transport remained unchanged (Figs4a, S5). Importantly, auxin transport activity further decreased to c. 25% of the wild-type level in the tt7 ugt78d2 double mutant, which accumulated even greater amounts of k1 (Figs3b, 4a). There was a dose-dependent, linear relationship between the k1 level and PAT, when comparing the wild-type, ugt78d2, tt7 and tt7 ugt78d2 (Fig.4b). The regulation did not affect the transcript level of genes encoding major, putative auxin export proteins, as mRNA levels of PIN1, PIN3, PIN7, ABCB1 and ABCB19 were not significantly different in wild-type and ugt78d2 stems (Fig. S6).
Figure 4.
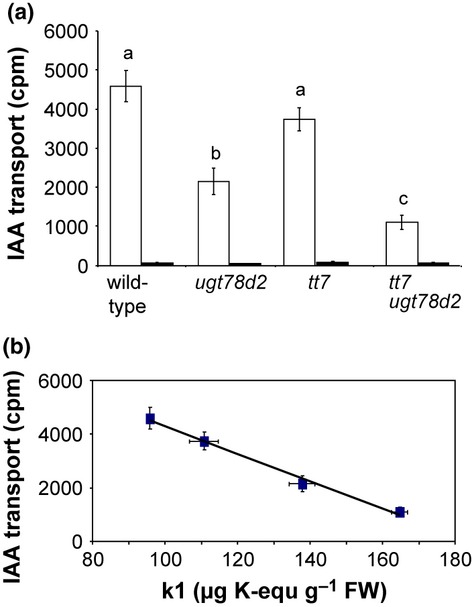
Basipetal auxin transport in inflorescence stems. (a) Basipetal auxin transport in Arabidopsis thaliana wild-type, ugt78d2, tt7 and tt7 ugt78d2 basal stem segments. Data represent radioactivity accumulated in basal segments (means ± SE obtained from 10 individual plants without N-1-naphthylphthalamic acid (NPA) treatment and from two individual plants for NPA treatment)). Closed bars, +NPA; open bars, –NPA. Letters above the error bars represent a significant difference between genotypes by a paired t-test (P < 0.05). (b) Basipetal auxin transport in relation to k1 levels. Mean value and standard error are plotted. A linear relationship was observed (R2 = 0.9911).
The altered PAT also raised the question of whether there was a general difference in auxin responsiveness. However, several auxin-inducible marker genes were up-regulated by exogenous IAA application in the same manner in ugt78d2 and the wild-type (Fig. S7).
Free IAA level is reduced in ugt78d2 inflorescence stems
To examine whether the reduced auxin transport influenced the concentration of free IAA in ugt78d2 stems, the free IAA contents of three consecutive stem segments of ugt78d2 and wild-type plants were compared. The endogenous levels of free IAA in the apical inflorescence segments were not different from those of the wild-type (P > 0.05), whereas the middle and basal stem segments of ugt78d2 contained lower steady-state IAA levels than the wild-type counterparts (P < 0.05; Table1). This suggested that the decreased IAA content in the lower parts of the inflorescence stem of ugt78d2 was caused by the reduced basipetal transport of auxin from sites of its biosynthesis in the apex.
Table 1.
Free indole-3-acetic acid (IAA) quantification in Arabidopsis thaliana inflorescence stem segments
| Apical segment | Middle segment | Basal segment | |
|---|---|---|---|
| Wild-type | 207 ± 50 | 253 ± 23 | 247 ± 27 |
| ugt78d2 | 194 ± 17 | 118 ± 14* | 142 ± 18* |
Free IAA was measured in three consecutive stem segments (pmol g−1 FW). The mean values ± SE of three independent biological samples (one sample is a pool of four to six individual stem segments) are displayed. Statistical analyses were performed by a paired t-test for uneven variance for each part of the stem between the two genotypes (*, P < 0.05).
Gravitropism of the inflorescence stem is delayed in ugt78d2 plants
The reduction of PAT in the inflorescence stems of ugt78d2 could also affect tropic responses. Therefore, gravitropism was analyzed in the mutant. Four-week-old plants were laid down horizontally in the dark to exert a gravistimulus, which would lead to an upward bending of the inflorescence stems. The bending angle of the inflorescence stem was monitored at different time points after the gravistimulus. ugt78d2 exhibited a strongly delayed gravitropic response, indicating that the repressed auxin transport was correlated with a reduced gravitropism in ugt78d2 inflorescence stems (Fig.5).
Figure 5.
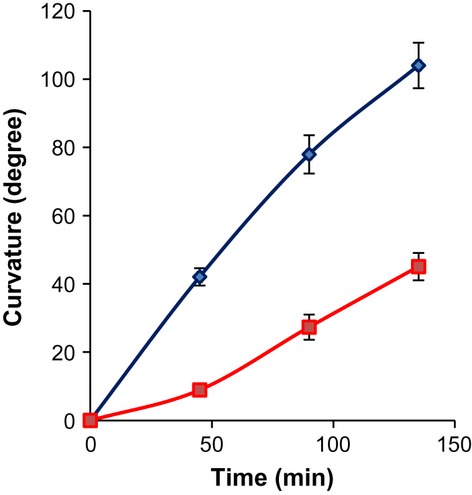
Gravitropic response of wild-type and ugt78d2 inflorescence stems. Four-week-old Arabidopsis thaliana plants were laid down from an upright to a horizontal position in darkness. The curvature of the primary inflorescence stems was measured at different time points to assess the gravitropic response. The angles refer to a horizontal positioning of the tip of the inflorescence (0°) and a fully upright position of the tangential approximation of the bent tip (90°). Wild-type, blue line; ugt78d2, red line. Means and SE are shown (n = 15).
Discussion
Over the past decade, strong evidence, both in vitro and in vivo, has accumulated implicating flavonols in plant growth regulation and auxin transport modulation (Jacobs & Rubery, 1988; Buer & Muday, 2004; Peer et al., 2004; Ringli et al., 2008; Santelia et al., 2008; Kuhn et al., 2011; Lewis et al., 2011; Grunewald et al., 2012; Buer et al., 2013). However, the physiologically active flavonol derivative(s) could not be pinpointed unequivocally. In vivo feeding studies were hampered by the possible modification and metabolism of the exogenously added compounds. Similarly, genetic studies affecting either flavonol biosynthesis or flavonol conjugation did not generate effects that could be traced back to a single flavonol moiety (Buer & Muday, 2004; Peer et al., 2004; Ringli et al., 2008; Buer et al., 2013). Nevertheless, the genetic identification of the kaempferol derivative k1 as an endogenous inhibitor of PAT in this work is in agreement with previous studies. These have indicated activity associated with exogenously applied flavonols that could be metabolized to k1 (Jacobs & Rubery, 1988; Mathesius et al., 1998). Furthermore, a release from PAT suppression in flavonol-free tt4 was observed, whereas PAT is further repressed in tt3, which exhibits an enhanced flavonol content at the expense of anthocyanins, and, importantly, in tt7, which shows specifically increased kaempferol glycoside levels at the expense of quercetins (Buer & Muday, 2004; Peer et al., 2004). Furthermore, the formation of k1 is dependent on the 3-O-rhamnosyltransferase UGT78D1, which is relatively abundant at the shoot apex in comparison with other tissues and almost absent from roots in agreement with a low k1 level in roots (Jones et al., 2003) (Figs S1, S8). Thus, the expression pattern of UGT78D1 correlates with an in situ activity of k1 in modulating auxin transport and affecting the shoot phenotype.
Nevertheless, the identification of k1 as an active compound does not exclude an impact of other flavonol derivatives on auxin fluxes. Flavonol biosynthesis is under developmental and organ-specific regulation by MYB and WRKY transcription factors (Stracke et al., 2007, 2010b; Grunewald et al., 2012). Accordingly, the active compounds could also be dependent on the developmental stage and/or on the organ. WRKY23 has been revealed to be part of a feedback loop of auxin to repress its own transport in roots, as it was induced by auxin and enhanced the biosynthesis of flavonols, in particular of quercetins. Thus, quercetins have been suggested to be active agents in roots (Grunewald et al., 2012; Buer et al., 2013). This finding was supported by the analysis of the quercetin-less tt7, which indicated that no kaempferol derivatives, but rather quercetins, were involved in the suppression of basipetal auxin flux in roots (Lewis et al., 2011; Grunewald et al., 2012). However, Buer et al. (2013) also found a repressed root PAT in both quercetin-accumulating tt3 and quercetin-deficient tt7. Nevertheless, these data and the very low k1 level in roots, as well as the lack of obvious ugt78d2-related phenotypes in hypocotyls and roots, including a similar auxin accumulation in mutant and wild-type root tips monitored by a DR5::β-glucuronidase reporter line, strongly support the notion that different flavonol compounds may affect PAT in root and shoot (Figs S1, S9; Table S1).
Suppression of rhamnose biosynthesis in rol1-2 strongly altered the flavonol profile and induced, for example, hyponastic growth of cotyledons and aberrant leaf cell development. This was related to enhanced auxin accumulation and a repressed auxin (non-IAA) efflux from mesophyll protoplasts (Ringli et al., 2008; Kuhn et al., 2011). As rol1-2 growth defects were rescued in tt4 rol1, but retained in tt7 rol1, they were attributed to kaempferols. However, k1 was not the causal metabolite for this particular phenotype, as rol1 ugt78d1 lacking k1 retained the rol1 cotyledon phenotype (Ringli et al., 2008).
Several possible mechanisms have been reported on how flavonols might affect auxin transport. Flavonol biosynthetic mutants led to an altered expression, subcellular localization and perhaps modified dynamics in the plasma membrane of some PIN proteins, which could have been caused by a direct or indirect impact of flavonols on these proteins (Peer et al., 2004; Santelia et al., 2008). At the transcriptional level, there was no difference in mRNA abundance of several PIN as well as ABCB1 and ABCB19 genes in ugt78d2 and wild-type plants (Fig. S6). ABCB1 and ABCB19 are ABC transporters which primarily function in the long-distance auxin transport streams and the movement of auxin out of apical tissues (Bandyopadhyay et al., 2007). Therefore, these ABCB transporters are potential targets of k1 in planta. Indeed, ABCB1/19 proteins are able to bind the quercetin aglycone (Murphy et al., 2002), and inhibition of ABCB1 auxin transport activity by the quercetin aglycone has been demonstrated in Arabidopsis protoplasts (Geisler et al., 2005). These observations are in agreement with previous in vitro experiments showing that flavonol aglycones can compete with the synthetic auxin transport inhibitor NPA for binding to isolated microsomal vesicles, although these results may not necessarily reflect an in vivo relevance (Jacobs & Rubery, 1988; Murphy et al., 2002). Nevertheless, these studies indicate that flavonol derivatives, including k1, could interact directly with and/or inhibit auxin transport proteins.
Despite these in vitro studies, a possible involvement of flavonol aglycones in auxin transport inhibition in vivo and, in particular, in the ugt78d2 growth phenotype appears to be unlikely. This notion is based on two lines of evidence: first, to the best of our knowledge, so far only two of numerous studies have detected flavonol aglycones in Arabidopsis by either HPLC (Peer et al., 2001) or mass spectrometry (Buer et al., 2013); however, the latter authors also discussed inconsistencies with respect to the occurrence and absence of aglycones in different tt mutants. Although flavonol aglycones exist at least as biosynthetic intermediates, only minute amounts of the hydrophobic molecules might be tolerated in living cells, and therefore free flavonols are not consistently detected; however, this would not preclude a regulatory role (Yin et al., 2012). The important second line of evidence stems from loss-of-function mutants affecting the two major UGTs in Arabidopsis, UGT78D1 and UGT78D2, which perform the initial 3-O-flavonol glycosylation (Fig.1). The lack of either UGT is compensated by the remaining one, and results in a shift of the flavonol glycoside pattern with no aglycones being detected (this work; Jones et al., 2003; Yonekura-Sakakibara et al., 2008; Yin et al., 2012). The ugt78d1 ugt78d2 double mutant strongly enhances the chance of flavonol aglycone accumulation, yet no free flavonols were detected (Yin et al., 2012) and, more importantly, the ugt78d2-dependent growth retardation was reversed in the double mutant (Fig.2).
Flavonols have also been shown to exert their function by modulating the interaction between TWD1, a regulatory protein, and ABCB1 transporters, thus suggesting another possible scenario (Bailly et al., 2008). Furthermore, it has been reported that PINOID modulates ABCB1-mediated auxin transport through its kinase activity, and that this effect is reversed by direct quercetin binding, that is, the flavonol acts as an endogenous kinase inhibitor to modulate PAT (Henrichs et al., 2012). In summary, these mechanistic studies on auxin transport inhibition allow for both different active flavonol moieties and multiple target proteins. The discovery of k1 as an active molecule in planta may serve as a valuable tool to obtain further insight into the detailed mechanism of flavonol-dependent auxin transport inhibition.
Apart from the genetic designation of k1, the dose-dependent PAT repression by k1 further supports its identification as an endogenous PAT inhibitor in Arabidopsis shoots (Fig. 4). Interestingly, there is also almost the same quantitative relationship in ugt78d2 relative to the wild-type between the concentrations of k1 (c. two-fold higher in the mutant), endogenous auxin (c. two-fold lower in the lower inflorescence stem segments of mutant plants) and basipetal auxin transport (c. two-fold lower in the basal part of the inflorescence stem of the mutant) (Figs3b, 4a; Table 4). A further increase in k1 in tt7 ugt78d2 resulted in more severe growth defects. However, the complete lack of k1 (in tt4, ugt78d1, ugt89c1) did not result in a higher stature of the A. thaliana plants, suggesting a threshold for k1-dependent growth repression or an independent limitation of growth of these mutants.
In plants, a minor increase in k1 levels would only lead to moderate PAT inhibition, which might not lead to obvious phenotypic changes (as seen in the shoots of abcb1 and abcb19 single mutants; Noh et al., 2001). However, under harsh natural growth conditions, including high light, UV-B irradiation, extreme temperatures and drought, k1 as well as other flavonol glycosides could be strongly increased (Hannah et al., 2006; Rausher, 2006; Caldwell et al., 2007; Korn et al., 2008; Götz et al., 2010; Stracke et al., 2010a). Thus, regulation of flavonol glycoside levels may constitute a means to adjust plant growth and stature by environmental factors and in ecological contexts.
Acknowledgments
We thank R. Stracke for providing tt4 and ugt89c1 seeds, J. Durner for helpful suggestions and R. Ulm, G. Bahnweg and L. Lin for critical reading of the manuscript. We are also grateful to two anonymous reviewers whose remarks helped to improve this study. B. Geist and S. Stich provided excellent technical assistance; S. Dräxl assisted with the auxin transport experiments. The work of P.I.D. and E.Z. was supported by the Grant Agency of the Czech Republic, project P305/11/0797.
Supporting Information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig S1Morphology and flavonol content of young seedlings.
Fig. S2 Genetic complementation of the ugt78d2-dependent shoot growth phenotype.
Fig. S3 Growth phenotype and k1 levels of fls1 ugt78d2.
Fig. S4 Growth phenotype and k1 levels of tt3-1 and Ler.
Fig. S5 Acropetal auxin transport activity in inflorescence stems of wild-type and ugt78d2.
Fig. S6 Expression of auxin transporter genes in ugt78d2 stem segments relative to wild-type.
Fig. S7 Auxin-responsive gene expression in ugt78d2 and wild-type.
Fig. S8 Analysis of UGT78D1 gene expression in plant organs.
Fig. S9 Expression of auxin reporter DR5::GUS in wild-type and ugt78d2 roots.
Table S1 Phenotypic analysis of Col-0 and ugt78d2 plants
References
- Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR. The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant Journal. 2003;35:624–636. doi: 10.1046/j.1365-313x.2003.01834.x. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Bailly A, Sovero V, Vincenzetti V, Santelia D, Bartnik D, Koenig BW, Mancuso S, Martinoia E, Geisler M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. Journal of Biological Chemistry. 2008;283:21817–21826. doi: 10.1074/jbc.M709655200. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Blakeslee JJ, Lee OR, Mravec J, Sauer M, Titapiwatanakun B, Makam SN, Bouchard R, Geisler M, Martinoia E, et al. Interactions of PIN and PGP auxin transport mechanisms. Biochemical Society Transactions. 2007;35:137–141. doi: 10.1042/BST0350137. [DOI] [PubMed] [Google Scholar]
- Berleth T, Scarpella E, Prusinkiewicz P. Towards the systems biology of auxin-transport-mediated patterning. Trends in Plant Science. 2007;12:151–159. doi: 10.1016/j.tplants.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta. 2013;238:171–189. doi: 10.1007/s00425-013-1883-3. [DOI] [PubMed] [Google Scholar]
- Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 2004;16:1191–1205. doi: 10.1105/tpc.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MM, Bornman JF, Ballare CL, Flint SD, Kulandaivelu G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochemical & Photobiological Sciences. 2007;6:252–266. doi: 10.1039/b700019g. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Deruère J, Jackson K, Garbers C, Soll D, Delong A. The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant Journal. 1999;20:389–399. doi: 10.1046/j.1365-313x.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Havlicek L, Vagner M, Malbeck J, Kaminek M. Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. Journal of Chromatography A. 2005;1075:159–166. doi: 10.1016/j.chroma.2005.02.091. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Vankova R. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Methods in Molecular Biology. 2012;913:251–261. doi: 10.1007/978-1-61779-986-0_17. [DOI] [PubMed] [Google Scholar]
- Friml J. Auxin transport – shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant Journal. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Götz M, Albert A, Stich S, Heller W, Scherb H, Krins A, Langebartels C, Seidlitz HK, Ernst D. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period. Protoplasma. 2010;243:95–103. doi: 10.1007/s00709-009-0064-5. [DOI] [PubMed] [Google Scholar]
- Grunewald W, De Smet I, Lewis DR, Lofke C, Jansen L, Goeminne G, Vanden Bossche R, Karimi M, De Rybel B, Vanholme B, et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proceedings of the National Academy of Sciences, USA. 2012;109:1554–1559. doi: 10.1073/pnas.1121134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiology. 2006;142:98–112. doi: 10.1104/pp.106.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichs S, Wang B, Fukao Y, Zhu J, Charrier L, Bailly A, Oehring SC, Linnert M, Weiwad M, Endler A, et al. Regulation of ABCB1/PGP1-catalysed auxin transport by linker phosphorylation. EMBO Journal. 2012;31:2965–2980. doi: 10.1038/emboj.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Motohashi R, Kuromori T, Noutoshi Y, Seki M, Kamiya A, Mizukado S, Sakurai T, Shinozaki K. A resource of 5,814 dissociation transposon-tagged and sequence-indexed lines of Arabidopsis transposed from start loci on chromosome 5. Plant and Cell Physiology. 2005;46:1149–1153. doi: 10.1093/pcp/pci112. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jones P, Messner B, Nakajima J, Schäffner AR, Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. Journal of Biological Chemistry. 2003;278:43910–43918. doi: 10.1074/jbc.M303523200. [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Korn M, Peterek S, Mock HP, Heyer AG, Hincha DK. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant, Cell & Environment. 2008;31:813–827. doi: 10.1111/j.1365-3040.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BM, Geisler M, Bigler L, Ringli C. Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiology. 2011;156:585–595. doi: 10.1104/pp.111.175976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Yoon HR, Paik YS, Liu JR, Chung WI, Choi G. Reciprocal regulation of Arabidopsis UGT78D2 and BANYULS is critical for regulation of the metabolic flux of anthocyanidins to condensed tannins in developing seed coats. Journal of Plant Biology. 2005;48:356–370. [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiology. 2011;156:144–164. doi: 10.1104/pp.111.172502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant Journal. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- Murphy AS, Hoogner KR, Peer WA, Taiz L. Identification, purification, and molecular cloning of N-1-naphthylphthalamic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiology. 2002;128:935–950. doi: 10.1104/pp.010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiology. 2001;126:536–548. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–1911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Blakeslee JJ, Yang H, Murphy AS. Seven things we think we know about auxin transport. Molecular Plant. 2011;4:487–504. doi: 10.1093/mp/ssr034. [DOI] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science. 2007;12:556–563. doi: 10.1016/j.tplants.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Rausher MD. The evolution of flavonoids and their genes. In: Grotewold E, editor. The science of flavonoids. New York, NY, USA: Springer; 2006. pp. 175–211. [Google Scholar]
- Ringli C, Bigler L, Kuhn BM, Leiber RM, Diet A, Santelia D, Frey B, Pollmann S, Klein M. The modified flavonol glycosylation profile in the Arabidopsis rol1 mutants results in alterations in plant growth and cell shape formation. Plant Cell. 2008;20:1470–1481. doi: 10.1105/tpc.107.053249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Molecular Biology. 2003;53:247–259. doi: 10.1023/B:PLAN.0000009297.37235.4a. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, et al. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. Journal of Biological Chemistry. 2008;283:31218–31226. doi: 10.1074/jbc.M710122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckmeyer G, Payer HD. A new sunlight simulator for ecological research on plants. Journal of Photochemistry and Photobiology B: Biology. 1993;21:175–181. [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant Journal. 1995;8:659–671. doi: 10.1046/j.1365-313x.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant, Cell & Environment. 2010a;33:88–103. doi: 10.1111/j.1365-3040.2009.02061.x. [DOI] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant Journal. 2007;50:660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Jahns O, Keck M, Tohge T, Niehaus K, Fernie AR, Weisshaar B. Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytologist. 2010b;188:985–1000. doi: 10.1111/j.1469-8137.2010.03421.x. [DOI] [PubMed] [Google Scholar]
- Thiel S, Dohring T, Kofferlein M, Kosak A, Martin P, Seidlitz HK. A phytotron for plant stress research: how far can artificial lighting compare to natural sunlight? Journal of Plant Physiology. 1996;148:456–463. [Google Scholar]
- Tohge T, Nishiyama Y, Hirai MY, Yano M, Nakajima J, Awazuhara M, Inoue E, Takahashi H, Goodenowe DB, Kitayama M, et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant Journal. 2005;42:218–235. doi: 10.1111/j.1365-313X.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- Tohge T, Yonekura-Sakakibara K, Niida R, Watanabe-Takahashi A, Saito K. Phytochemical genomics in Arabidopsis thaliana: a case study for functional identification of flavonoid biosynthesis genes. Pure Applied Chemistry. 2007;79:811–823. [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Current Biology. 2006;16:1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Yin R, Messner B, Faus-Kessler T, Hoffmann T, Schwab W, Hajirezaei MR, von Saint PaulV, Heller W, Schäffner AR. Feedback inhibition of the general phenylpropanoid and flavonol biosynthetic pathways upon a compromised flavonol-3-O-glycosylation. Journal of Experimental Botany. 2012;63:2465–2478. doi: 10.1093/jxb/err416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Tohge T, Matsuda F, Nakabayashi R, Takayama H, Niida R, Watanabe-Takahashi A, Inoue E, Saito K. Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene–metabolite correlations in Arabidopsis. Plant Cell. 2008;20:2160–2176. doi: 10.1105/tpc.108.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Tohge T, Niida R, Saito K. Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. Journal of Biological Chemistry. 2007;282:14932–14941. doi: 10.1074/jbc.M611498200. [DOI] [PubMed] [Google Scholar]
- Zažímalová E, Murphy AS, Yang HB, Hoyerová K, Hošek P. Auxin transporters – why so many? Cold Spring Harbor Perspectives in Biology. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1Morphology and flavonol content of young seedlings.
Fig. S2 Genetic complementation of the ugt78d2-dependent shoot growth phenotype.
Fig. S3 Growth phenotype and k1 levels of fls1 ugt78d2.
Fig. S4 Growth phenotype and k1 levels of tt3-1 and Ler.
Fig. S5 Acropetal auxin transport activity in inflorescence stems of wild-type and ugt78d2.
Fig. S6 Expression of auxin transporter genes in ugt78d2 stem segments relative to wild-type.
Fig. S7 Auxin-responsive gene expression in ugt78d2 and wild-type.
Fig. S8 Analysis of UGT78D1 gene expression in plant organs.
Fig. S9 Expression of auxin reporter DR5::GUS in wild-type and ugt78d2 roots.
Table S1 Phenotypic analysis of Col-0 and ugt78d2 plants


