Fig. 5.
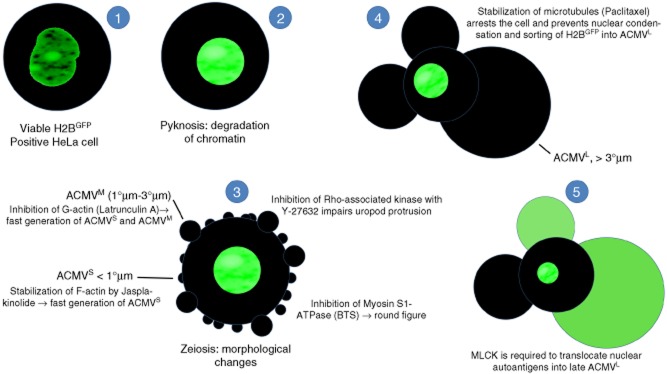
Synopsis: schematic display of the translocation in apoptotic cells of nuclear autoantigens. HeLa cells were transfected with pBOS-histone 2B-green fluorescent protein (H2BGFP) to label histone H2B as a representative of nuclear proteins serving as autoantigens in patients with systemic lupus erythematosus (SLE). After induction of apoptosis via irradiation with ultraviolet (UV)-B, processing and translocation of H2B was monitored employing fluorescence microscopy. The transfected cells were incubated with inhibitors of cell dynamics to identify the cytoskeletal proteins involved in the trafficking of H2B. (1) Viable H2BGFP expressing cell with a regularly configured nucleus. (2) Apoptosis starts with chromatin condensation and degradation (pyknosis). (3) In early apoptosis, the cells undergo dramatic morphological changes. They shrink and generate apoptotic cell-derived membranous vesicles (ACMV), zeiosis. Initially small (< 1 μm) and intermediate (1–3 μm) ACMVS/M are generated. These ACMV do not contain substantial amounts of H2BGFP. Several inhibitors of cell dynamics have impact on the morphology of the dying cell. p160ROCK (Y-27632) is required for uropod protrusion. Inhibition of F-actin (jasplakinolide) or G-actin (latrunculin A) leads to the early generation of ACMVS and ACMVM. Inhibition of myosin S1 ATPase [N-benzyl-p-toluene sulphonamide (BTS)] causes rounding of the cell. (4) However, all the above listed inhibitors do not have any impact on formation of late ACMVL and the translocation of H2B into these. Inhibition of microtubules (paclitaxel) arrests the apoptotic process at an early time-point. It impairs nuclear shrinkage as well as formation of ACMVL and translocation of H2BGFP. (5) Inhibition of myosin light chain kinase (MLCK) by 1-(5-chloronaphthalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine (ML-9) does not preclude the formation of ACMVL, but impairs their loading with histone H2BGFP.
