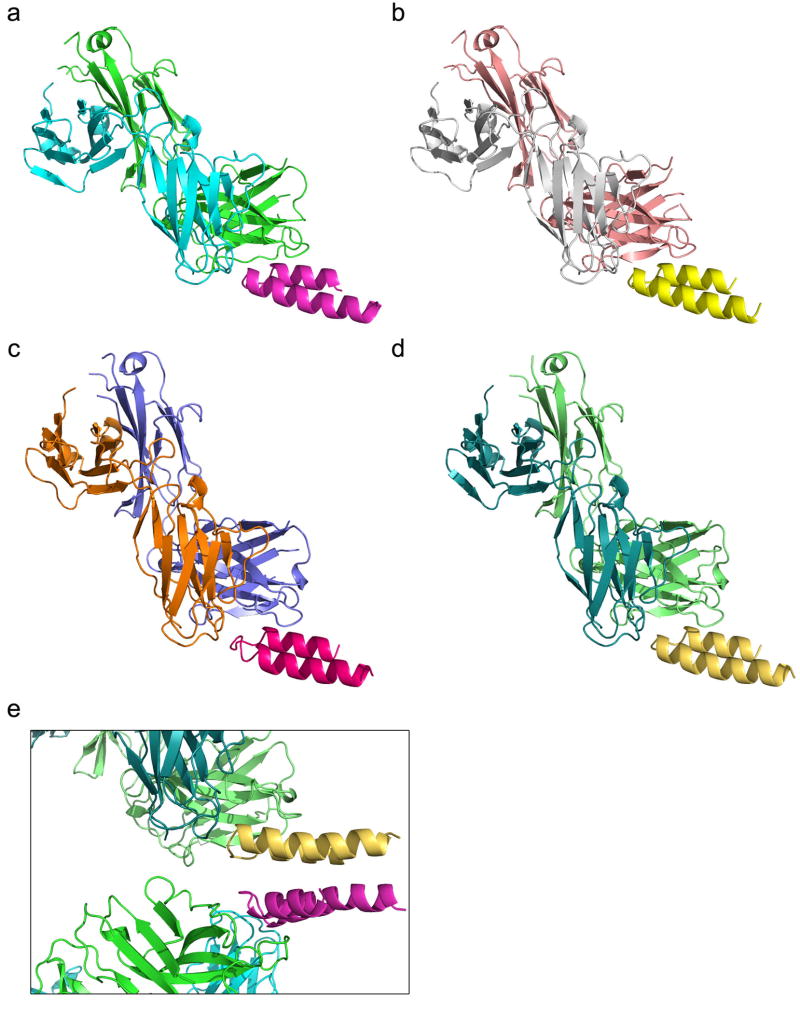
Extended Data Figure 10.
Four complex structures of 17-HD9+peptide in the asymmetric unit, from PDB: 4N9G. The four complexes in the asymmetric unit consisted of two pairs of nearly identical structures (RMSD within each pair was 0.3 Å), with the pairs differing from each other primarily in the Fv angle of approach to the epitope (angle difference ∼9°) and in the Fab elbow angle (angle difference ∼10°); differences within the peptide between pairs were small (RMSD over peptide between pairs was 0.7 Å). a) Chains A+B+C; b) chains E+F+D; c) chains H+L+Y; d) chains M+N+Z. e) View of crystal packing interaction, in which the “backside” of one peptide interacts with the “backside” of another. Partial scaffolds (peptides) are packed against each other at crystal contacts between complexes through an interface outside of the epitope, with perfect dyad symmetry broken by a translation along the NCS dyad axis to accommodate complementary packing of apolar side-chains. The crystal packing is incompatible with the scaffold being present as a three helix bundle as in the Mota or 31-HG7 complex structures. Clear density was lacking for the scaffold outside the helix-turn-helix peptide. Scaffold missing density is possibly due to partial proteolysis or unfolding of the scaffold that may have occurred while purified Fab+scaffold complexes incubated at high concentration (∼10 mg/mL) in crystallization liquor for three months prior to crystal formation (see Supplementary Methods). The location and size of solvent channels in the crystal could accommodate the disordered region of the scaffold as an extended, flexible peptide unfolded under the conditions of crystallization, but it is also plausible that limited proteolysis has reduced the scaffold to a minimal structure protected by contacts with the antibody.