Abstract
NMDA receptors (NMDAR) are important in the development and maintenance of central sensitization. Our objective was to investigate the role of spinal neurons and NMDAR in the maintenance of chronic visceral pain. Neonatal rats were injected with acidic saline adjusted to pH4.0 in the gastrocnemius muscle every other day for 12 days. In adult rats, NR1 and NR2B subunits were examined in the lumbo-sacral (LS) spinal cord. A baseline, visceromotor response (VMR) to graded colorectal distension (CRD) was recorded before and after administration of the NMDA antagonist, CGS-19755. Extracellular recordings were performed from CRD-sensitive LS spinal neurons and pelvic nerve afferents (PNA) before and after CGS-19755. Rats that received pH 4.0 saline injections demonstrated a significant increase in the expression NR2B subunits and VMR response to CRD >20mmHg. CGS-19755 (i.v. or i.t.) had no effect in naïve rats, but significantly decreased the response to CRD in pH4.0 saline injected rats. CGS-19755 had no effect on the spontaneous firing of SL-A, but decreased that of SL-S. Similarly, CGS-19755 attenuates the responses of SL-S neurons to CRD, but had no effect on SL-A neurons or on the response characteristics of PNA fibers. Neonatal noxious somatic stimulation results in chronic visceral hyperalgesia and sensitizes a specific subpopulation of CRD-sensitive spinal neurons. The sensitization of these SL-S spinal neurons is attenuated by the NMDAR antagonist. The results of this study suggest that spinal NMDARs play an important role in the development of hyperalgesia early in life.
Keywords: Visceral hyperalgesia, NMDA, Neonatal pain
1. Introduction
The excitatory amino acid, glutamate, is a major neurotransmitter in the mammalian central nervous system and plays an important role in nociceptive processing. Glutamate acts on two ionotropic receptor subtypes including NMDA (N-methyl-D-aspartate) and AMPA/kainite that are involved in plasticity, brain development, learning, excitatory synaptic transmission and long-term potentiation (Banerjee et al., 2009; Park et al., 2013; Yoshimura et al., 2013).
The “Wind–up” of spinal neurons to somatic nociceptive stimuli and long-term potentiation due to synaptic plasticity is believed to be largely mediated by NMDARs (Boxall et al., 1996; Davis and Lodge, 1987; Dickenson and Sullivan 1987; Randic et al., 1993; Liu and Sandkuhler 1995). The NMDAR is composed of 2 types of homologous membrane-spanning subunits, NR1 and NR2 (Gonda et al., 2012; Laube et al., 1997). NR2 can be classified into NR2A, NR2B, NR2C, and NR2D subunits. The NR2B subunit has been shown to be present in the forebrain and superficial lamina of the spinal cord where it is believed to play an important role in chronic sensitization (Li et al., 2011; Li et al., 2012). Multiple studies have shown that blocking the NMDAR reduces pain transmission to noxious stimuli and chronic pain in humans and in animals (Becerra et al., 2009, Costroman and Ness, 2002; Peles et al., 2004). However, the role of the NMDAR in chronic visceral hyperalgesia following early life pain has been understudied.
Animal models have demonstrated a critical time during development in which the spinal cord is vulnerable to permanent structural and functional alterations in pain pathways (Lidow et al., 2001; Ren et al., 2004; Traub et al., 2004; Virgo et al., 2000). We have previously shown the development of chronic visceral hyperalgesia in a model of early life somatic pain. Nociceptive somatic stimulation in the form of low pH saline injections in the gastrocnemius (GN) muscle of neonatal rats results in chronic sensitization of spinal neurons and somatic and visceral hyperalgesia (Miranda et al., 2006). Further, in adult rats that received the neonatal stimulus, short latency sustained (SL-S) neurons demonstrate a permanent increase in spontaneous firing and response to mechanical distension of the colon, whereas short latency abrupt (SL-A) neurons remain unchanged. Others have reported a similar effect on SL-S neurons in a model of visceral hyperalgesia induced by acute inflammation of the colon (Ness and Gebhart, 2000). It has also been suggested that these subtypes of neurons respond differently to pharmacological intervention (Ji and Traub 2002; Kozlowski et al., 2000).
We hypothesized that the NMDAR plays an important role in early sensitization of spinal neurons. Using a neonatal model of viscero-somatic convergence, we aimed to: 1) investigate alterations in the expression of the NMDAR subtypes in the spinal cord, 2) examine the effects and site of action of CGS-19755 (cis-4-phosphonomethyl-2-piperidine carboxylate), a competitive NMDAR antagonist, on visceral hyperalgesia, and 3) determine the effect of the NMDAR antagonist on different subtypes of CRD-sensitive spinal neurons and pelvic nerve afferents (PNA).
2. Materials and methods
2.1. Animals
The study was carried out in male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA). Rats were kept in controlled conditions with a 12 hour light/dark schedule and had access to both food and water ad libitum. Twenty-four hours before surgery the animals were placed in a wire bottom cage and access to food, but not water, was denied in order to empty the stomach. All experiments were approved by the Animal Care and Use Committee at the Medical College of Wisconsin and are in accordance with the International Association of Pain policies on use of laboratory animals.
2.2. Neonatal Acid Injections
Neonatal rats (postnatal day 8) received unilateral injections of pH 4.0 saline (0.1 ml) to the GN muscle. The injections were given every other day for 12 days (total of 6 injections). Rat pups demonstrated occasional vocalization only during the time of injection. No subsequent shaking or licking of the paws was noted. Because we have shown that needle prick only without injection of saline does not alter sensitivity, only naïve rats were used as control.32 The control rats were taken out of their cage and handled similarly to the experimental group during the 12 days, but without injections. Total duration of separation from mothers during the injections was no more than 60 s. All neonates were allowed to grow to 2 months of age without external interference and then underwent surgery for behavioral testing.
2.3. NMDAR Expression (RT PCR)
RT-PCR analysis was performed in spinal cord tissues to examine mRNA expression level of NMDA receptor subunits NR1 and NR2B in adult rats with neonatal pH 4.0 injections and control (naïve) animals. In both groups, the L6-S1 spinal cord segment from 2 month old rats was removed and total RNA extraction and cDNA preparation was carried out as previously described.2 The primer sequences used in this study were NR1(forward) 5’GGCAGTAAACCAGGCCAATA 3’; NR1(reverse) 5’ GTGGGAGTGAA-GTGGTCGTT 3’, NR2B (forward) 5’CC-AAGAGGAGGAAACAGCAG 3’; NR2B (reverse) 5’.TGAGGCGAGTTCTCCTTTGT 3’; GAPDH(forward) 5’ CCTGCCAAGTATGATGAC 3’, GAPDH(reverse) 5’ GGAGTTGCTGTTGAAGTC 3’. The PCR reactions were performed using iQ SYBR Green Supermix (BIORAD), 5 pmole forward and reverse primers and 2 μl cDNA from each tissue sample as template in a total of 25 μl of the reaction mixture. PCR program was an initial incubation for 3 min at 95°C followed by 45 cycles of amplification with each of 30 s at 95°C and 30 s at 57°C. To verify the amplification efficiency within each experiment, a serial dilution of cDNA derived from a RNA pool of control tissues was amplified in triplicate in each plate. The specificity of PCR reaction and possibility of primer dimerization were verified using melt curve program and no template control for each PCR reaction. As all PCR reactions were performed with equal efficiencies, relative mRNA expression level of the target gene was directly normalized against the expression level of reference gene GAPDH for the same tissue sample. The CT values for reference gene GAPDH was highly reproducible between samples and between PCR reactions. The cDNA preparation with CT values <35 was considered as specific implication and reactions with CT values >35 were not included in the study.
2.4. Surgery for Colon Sensitivity Testing
All surgical procedures were performed in adult rats under deep anesthesia with sodium pentobarbital (50 mg/kg −1I.P.). A pair of teflon-coated electrodes (Cooner wire, part no. A5631) was implanted in the external oblique muscle for electromyography (EMG) recordings. The pair of electrodes was externalized subcutaneously and protected using a siliconized tube sutured to the dorsal aspect of the neck. Postoperatively, animals were given subcutaneous buprenorphine hydrochloride (0.1 mg/kg −1) to relieve pain. Enrofloxacin (50mg/kg, I.M.) was given in the contralateral thigh preoperatively in all animals to prevent infection. Rats were housed separately following surgery and allowed to recover for 7 days before testing.
2.5. Colon Sensitivity Testing
Electromyographic recordings to measure contraction of the abdominal wall musculature during graded CRD were used to assess colonic sensitivity. This is known as the visceromotor response (VMR) (Ness and Gebhart 1991a, 1991b). To prevent excessive movement during recordings, individual rats were trained to stay in a Bollman cage for 1 hour for three consecutive days. Prior to recordings, a distensible latex balloon (5 cm in length) attached to a distension device through polyethylene tubing was inserted into the rectum. The balloon was kept in place by taping the catheter to the tail. A pressure transducer monitored the intralumial pressure during distensions (10, 20, 30, 40, and 60 mmHg). The pressures were held constant during the 30 s stimulus period, with a 180 s interstimulus interval. The EMG signal from the external oblique muscle was amplified through a low-noise AC differential amplifier (model 1700, A-M Systems, Inc.) and recorded on-line using the Spike 2/CED 1401 data acquisition program (CED 1401; Cambridge Electronic Design, Cambridge, UK).
Following baseline recordings, the effect of the NMDAR antagonist CGS-19755 was tested on the VMR of adult rats in the neonatal pH 4.0 saline injected and controls. In both groups, the drug was given intravenously (30μmol, i.v.) or intrathecally (20nmol, i.t.) to test the site of action. The SRF to graded CRD (10–60 mmHg) was recorded before and 10 min after CGS-19755 administration. The doses used were obtained from previous experiments (Miranda et al., 2004).
2.6. Surgery for Spinal and PNA Recordings
Surgical preparations for electrophysiological recordings have been previously described (Mickle et al., 2010). Briefly, prior to experiments, rats were deprived of food, but not water, for 12-26 h. Following sodium pentobarbital induction (50 mg/kg, i.p.), anesthesia was maintained with a constant intravenous infusion of 5–10 mg/kg/h through a right femoral vein catheter. The left carotid artery was also cannulated to monitor blood pressure. The rats were paralyzed with an initial dose of gallamine triethiodide (Flaxedil, Sigma, St. Louis, MO, USA) (10 mg/kg, i.v), and mechanically ventilated with room air (~60 cycles min −1) through a tracheal tube. The body temperature was kept within the physiological range with an overhead lamp.
For spinal cord recordings, the lumbosacral (L6-S1) spinal cord was exposed by laminectomy after placing the head in a stereotaxic head holder. The dura membrane was removed and a 1–2 cm saline-soaked gelatin sponge (Gelfoam, Pharmacia Upjohn Company, Michigan, MI, USA) was used to cover the exposed spinal cord. The exposed surface of the spinal cord was covered with warm mineral oil (37°C).
PNA surgery has also been extensively described (Miranda et al., 2011). Following anesthesia, an abdominal incision was made and the pelvic nerve was isolated from the surrounding fatty tissues. A pair of Teflon-coated stainless wires was placed around the nerve to deliver electrical stimulation. This was used to confirm that recordings were made from the dorsal sacral root. After securing the electrodes and draping a piece of peritoneal fat tissue around it, the abdomen was closed with silk sutures. PNA projections in the S1 dorsal root were identified by recording the evoked response to electrical stimulation (5–10 mv, 0.5 ms square wave pulse).
2.7. Spinal and PNA Recordings
Extracellular single-unit recordings from the lumbo-sacral (LS) spinal cord have been previously described in detail (Mickle et al., 2010). Stainless-steel microelectrodes (3–4 MΩ, FHC, Bowdoinham, ME, USA) were used to record from the LS segment (L6-S1) spinal segments. The placement of the electrode was 0.1–1.0 mm lateral to the spinal mid-line and 0.6– 1.8 mm ventral to the dorsal surface. Recordings from the LS spinal segment (L6-S1) examined populations of neurons in the two groups. The neurons were classified as either SL-A or SL-S based on their response characteristics to CRD (Ness and Gebhart, 1987). SL-A neurons demonstrate increased firing during CRD with an abrupt cessation of response after termination of the distending stimulus, while the SL-S neurons maintain a sustained response (> 4 s) following the distending stimulus. Neurons that responded to CRD and tail rotation were considered proprioceptive and were not included in the study.
For spinal and PNA recordings, the action potentials were amplified through a low-noise AC differential amplifier (model 3000; A-M Systems) and continuously monitored and displayed on an oscilloscope. A dual window discriminator (model DDIS-1; BAK Electronics) was used to discriminate the action potentials and to convert the signal to a rectangular TTL pulse. A distensible latex balloon (5 cm) attached to polyethylene tubing was inserted through the anus into the distal colon. The balloon was and held in place by taping the tubing to the tail. Colorectal distension-sensitive neurons (spinal or PNA) were identified using brief CRD as the search stimulus (40 mmHg). Once identified, the spontaneous activity and response to graded CRD (10, 20, 30, 40, and 60 mmHg of 30 s duration and with 3 min interstimulus time intervals) was recorded for each neuron. Action potentials, intracolonic pressure and blood pressure were recorded on-line with the Spike 2/CED 1401 data acquisition system (Cambridge Electronic Design). Wave-Mark analysis of the Spike 4 software (Cambridge Electronic Design) was used to distinguish individual action potentials following experiments.
The effect of the CGS-19755 on spinal neurons and PNAs was also examined in a separate set of animals. CRD-sensitive LS spinal neurons from pH 4.0 saline injected rats were identified and SRFs to graded CRD were constructed before and 10 min after CGS-19755 injection (30 μmol, i.v.). Similarly, a baseline SRF to graded CRD of PNAs was constructed in pH 4. 0 injected rats. The SRF was repeated 10 min after injection of CGS-19755 (30 μmol, i.v.).
2.8. Data Analysis
Statistical analysis was performed using SigmaStat (V2.03, SPSS Inc, Chicago, IL). For RT-PCR, results were expressed as relative mRNA expression in terms of CT values in relation to the amount of reference gene mRNA expression using formula = 2−CTtarget-CTGAPDH. The area under the curve of the EMG recordings was measured and averaged for each distension pressure. To compare pre and post CGS-19755, the data were normalized by dividing individual values by the value recorded at baseline (60mmHg distension). Comparison between groups was made using a Student t-test (P<0.05). Spinal neurons’ baseline firing and responses to CRD were calculated by measuring the total mean firing frequency before and during CRD (30sec). The firing frequency during the resting period was subtracted from the firing frequency during distension to determine actual response during the distension. Data are expressed as means ± S.E.M. and were analyzed at each distension pressure using one-way repeated measures ANOVA.
3. Results
3.1. NMDAR Expression
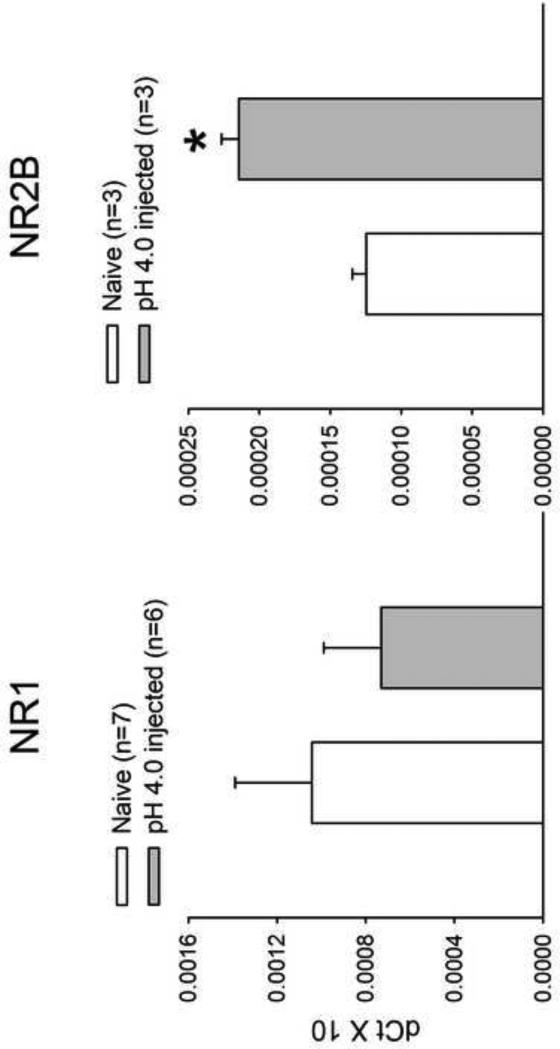
In order to first investigate whether the NMDA receptor was involved in the development of visceral hyperalgesia following neonatal somatic stimulation, the expression of the NR1 and NR2B subunits was measured in the L6-S1 spinal cord segments using real-time PCR. Adult rats that received pH 4.0 saline injections during the neonatal period demonstrated a significant increase in the expression NR2B subunits compared to controls (P<0.05). The NR1 expression in pH 4.0 saline rats was not increased compared to control rats (Fig.1).
Fig. 1.
Expression of the NR1 and NR2B subunits of the NMDAR from L6-S1 spinal segment using real-time PCR. Compared to controls, adult rats that received low pH saline injections during the neonatal period demonstrated a significant increase in the expression NR2B subunits and not NR1 (*P<0.05).
3.2. Visceral sensitivity to colorectal distension
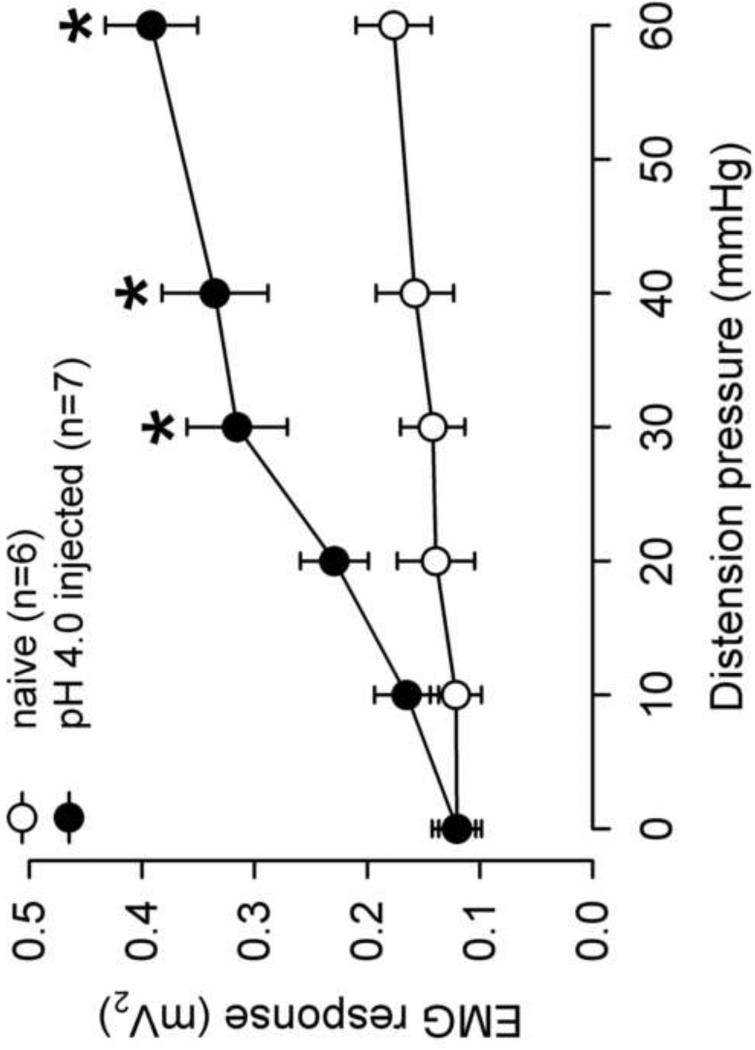
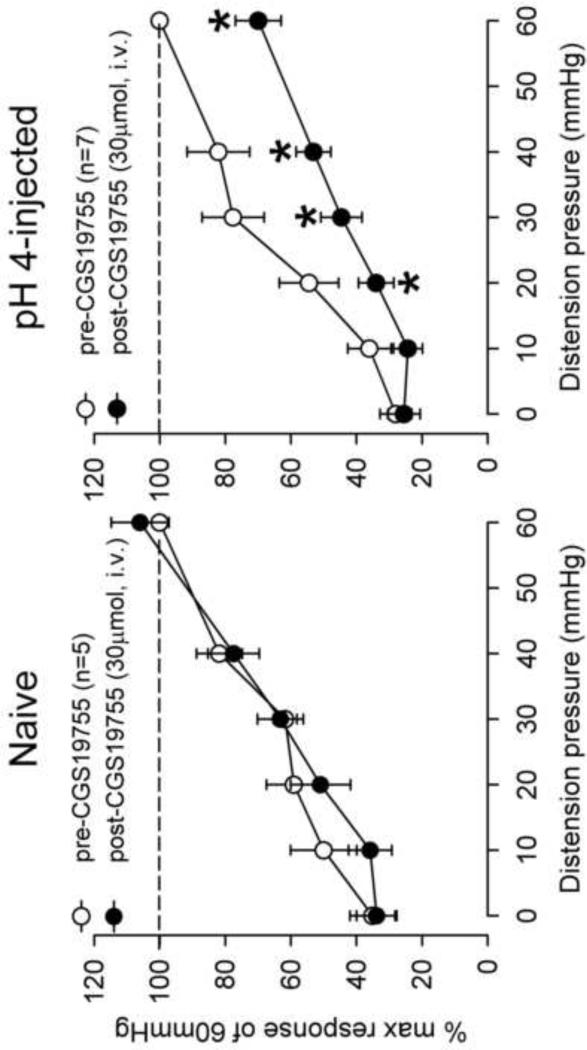
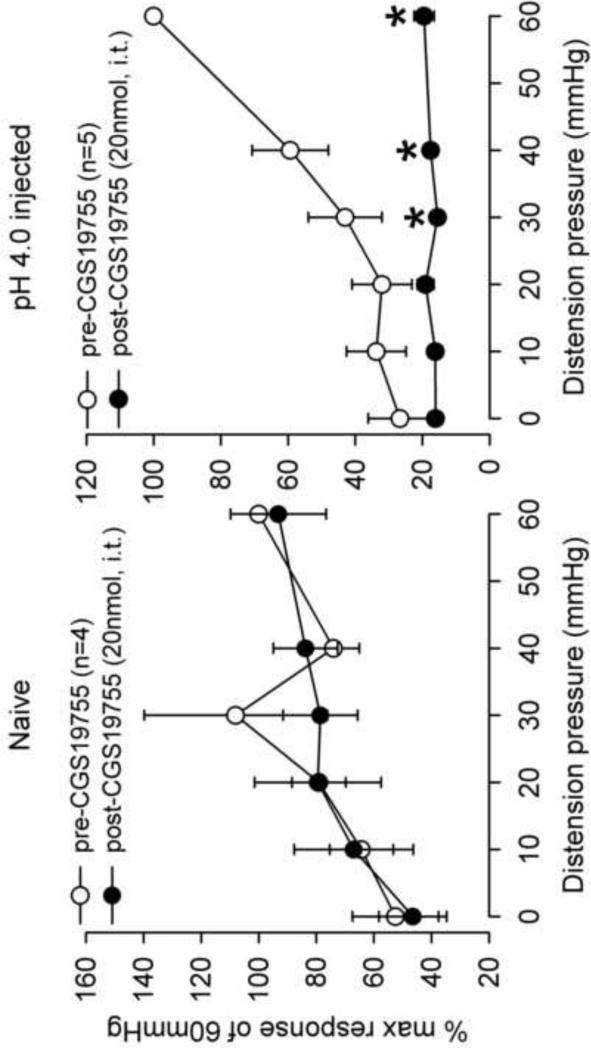
The visceromotor response to CRD was assessed in adult rats that received neonatal pH 4.0 saline injections and controls. There was a significant increase in the response to CRD pressures>30mmHg in the neonatal pH 4.0 sensitized rats compared to controls (Fig. 2). The effect of the NMDAR antagonist, CGS-19755 was first investigated via intravenous route. A SRF to graded CRD (10–60 mmHg) was recorded before and 10 min following CGS-19755 injection in both group (Fig 3A). There was no effect of CGS-19755 (30μmol) on the response to CRD in control rats. Conversely, the same dose significantly decreased the response to CRD pressures ≥20 mmHg in pH 4.0 saline treated rats (P<0.05, Fig 3B). Similarly, intrathecal injection of CGS-19755 (20nmol) had no effect on the VMR in of controls rats at all distension pressures tested but, significantly attenuated the response to CRD in pH 4.0 saline treated rats at pressures >20mmHg (Fig 4A and B).
Fig. 2.
Summary data of the VMR in naïve and pH 4.0 saline injected rats. The pH 4.0 saline injected rats demonstrated a greater response to mechanical distension compared to control at CRD pressures > 20mmHg (*P<0.05). This represents the development of visceral hyperalgesia in adult rats following neonatal pH 4.0 injections.
Fig. 3.
The mean VMR to graded CRD before and after intravenous CGS-19755 in naïve and pH 4.0 saline injected rats. CGS-19755 (30 μmol, i.v.) had no effect on the VMR of naïve rats (A). In pH 4.0 saline injected rats, there was a significant decrease in response to CRD pressures >10 mmHg (B) (*P < 0.05 vs pre-CGS).
Fig. 4.
The mean VMR to graded CRD before and after intrathecal CGS-19755 in naïve and pH 4.0 saline injected rats. CGS-19755 (20 nmol, i.t.) had no effect on the VMR of naïve rats (A). In pH 4.0 saline injected rats, there was a significant decrease in response to CRD pressures >40 mmHg (B) (*p < 0.05 vs pre-CGS).
3.3. Spinal Neurons
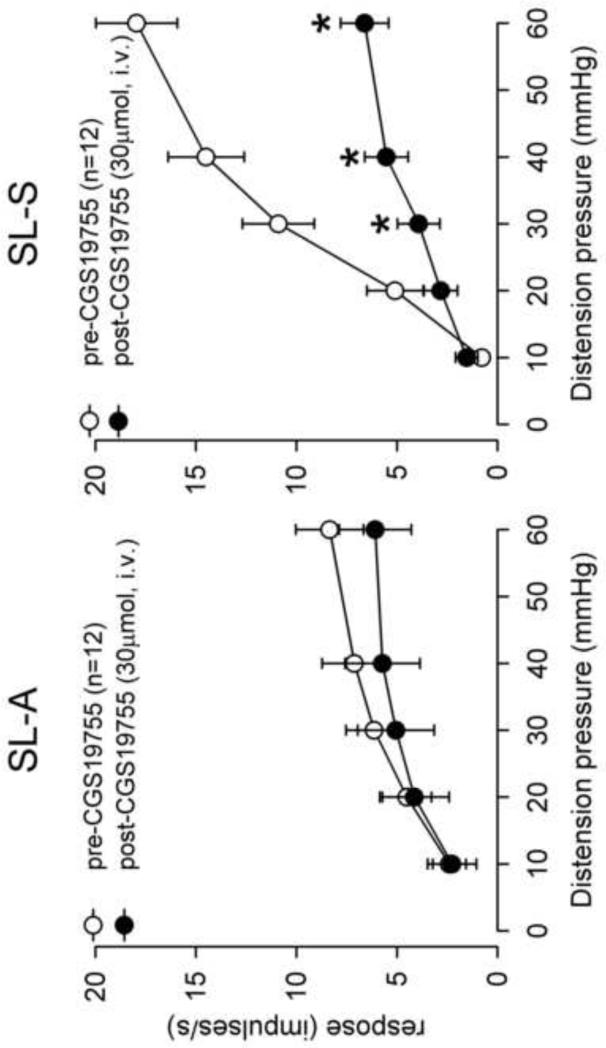
A total of 24 neurons in the L6-S1 spinal region were recorded from neonatal pH 4.0 saline injected rats and tested to graded intensities of CRD. Of these, 12 were SL-A and 12 were SL-S. The spontaneous firing for SL-A and SL-S neurons was 5.2±0.9 and 15.5±2.0 impulses/sec, respectively (P<0.05). We have previously shown that SLS neurons, and not SLA, are sensitized in adult rats following neonatal pH 4.0 saline injections with a higher spontaneous firing and increased response to CRD (Miranda et al., 2006). CGS-19755 (30μmol, i.v.) had no effect on spontaneous firing of SL-A (5.2±0.9 to 4.7±0.8) (P>0.05) but significantly decrease the spontaneous firing of SL-S neurons (15.5±2.0 to 5.3±1.4), (P<0.05). Similarly, in pH 4.0 saline injected rats, CGS-19755 had no effect on the response characteristics of SL-A spinal neurons to CRD (Fig. 5A). However, there was a significant reduction in the response of SL-S neurons to CRD following CGS-19755 injection at pressures >20 mmHg (Fig. 5B). Fig. 6 shows an example of a single SL-S neuron at different CRD pressures before and after CGS-19755.
Fig. 5.
The mean SRFs of SL-A and SL-S spinal neurons from pH 4.0 saline injected rats before and after CGS-19755 (30 μmol, i.v.). The spontaneous firing of each neuron was subtracted from the firing frequency during distension to determine the actual response. CGS-19755 had no effect on the response to CRD of SL-A spinal neurons (A). The response of SL-S neurons to CRD was significantly decreased by CGS-19755 at pressures >20 mmHg (P<0.05) (B).
Fig. 6.
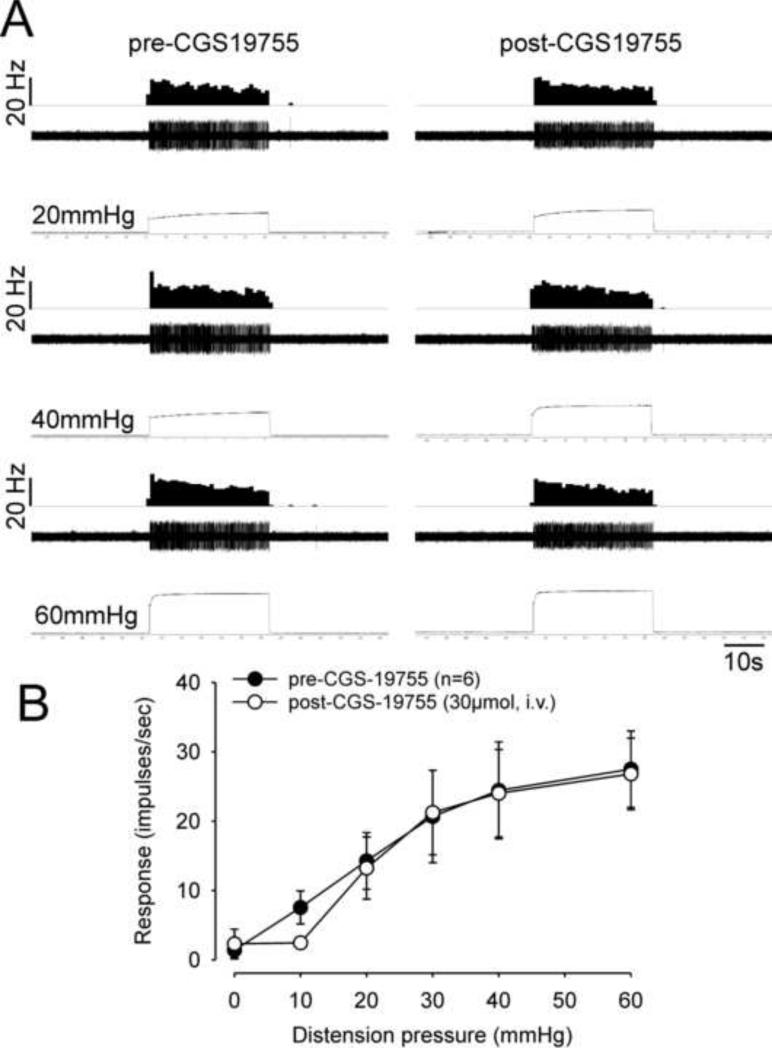
Example of the typical response of a CRD-sensitive SL-S spinal neuron in a pH 4.0 saline injected rat before and after CGS-19755. In all panels, the top trace shows the response to CRD represented as a frequency histogram (1 s bin width), the middle trace is the neuron action potential and the bottom trace is the distension pressure.
3.4. PNA fibers
In order to examine the involvement of primary sensory neurons and the site of action of CGS-19755, we recorded mechanosensitive PNA fibers to graded CRD in adult rats following neonatal pH 4.0 saline injections. CGS-19755 administration did not change the spontaneous firing of PNA fibers (0.07 ± 0.7 vs 0.03 ± 0.3 mean impulses/s, P>0.05) or their response to CRD, suggesting that the effect of CGS-19755 is not via primary sensory neurons (Fig. 7). Since CGS-19755 had no effect on the VMR to CRD in control rats, only PNA fibers of rats that received pH 4.0 saline injections were tested in this study.
Fig. 7.
The mean stimulus–response functions of CRD-sensitive pelvic nerve afferents from pH 4.0 saline injected rats before and after CGS-19755 showed that the NMDAR antagonist had no effect on responses to graded CRD (P>0.05).
4. Discussion
In the present study, we showed that early neonatal somatic pain results in chronic visceral hyperalgesia that persists into adulthood. We have demonstrated that: 1) the hyperalgesia is associated with an up-regulation of the NR2B subunits in the L6-S1 spinal cord, 2) intrathecal administration of a NMDAR antagonist, attenuates the visceral sensitivity only in sensitized rats, and 3) the NMDA antagonist alters the response characteristics of sensitized SL-S neurons in the L6-S1 spinal cord, but has no effect on SL-A neurons or PNAs.
The data highly suggest that the underlying chronic visceral hypersensitivity resulting from neonatal somatic stimulation involves NMDARs in areas of viscerosomatic convergence. The involvement of these receptors in the development of hyperalgesia and allodynia has been well documented in the literature (Gaudreau and Plourde, 2004; Gaunitz et al., 2002; Li et al., 2006; Ren and Dubner 1993; Ultenius et al., 2006; Urch et al., 2001; Willert et al.,2004). These receptors have been shown to undergo plastic changes during normal development and in pathological conditions (Monyer et al., 1994; Sheng et al., 1994). In a recent publication, we demonstrated the development of chronic colonic hyperalgesia following neonatal cystitis that was associated with an increase in the spinal NR1 subunit expression (Miranda et al., 2011). The expression of NR1 and NR2B subunits, both at the mRNA and protein level, is also increased in the rat lumbar spinal cord following neonatal nerve crush injury (Virgo et al., 2000). The models of early life pain and the role of NMDA in maintaining hypersensitivity states is particularly interesting, especially since, the NMDAR subunit (NR1, NR2A and NR2B) expression in the rat spinal cord is known to be high during the first three weeks after birth and then decreases to normal levels as the animal approaches adulthood (Brown et al., 2002). Thus, higher subunit expression may have functional consequences, particularly during development in the presence of pain. Increased expression of NR2B in a model of visceral hyperalgesia could affect NMDAR internalization and stability of synapses (Fan et al., 2009). Interestingly, Fan et al. found an increase in NR2B subunit expression without an increase in NR1 in the anterior cingulate cortex of rats that demonstrated visceral hyperalgesia. Similarly, in the current study, only NR2B expression remained high in sensitized rats compared to controls. It has been suggested that up-regulated NR2B is able to form functional NMDA receptors with NR1 that is usually in excess of NR2B, however, this remains speculative (Fan et al., 2009). Overall, multiple studies have proposed NR2B as the major subunit involved in the development and maintenance of hyperalgesia (Abe et al., 2005; Iwata et al., 2007; Geng et al., 2010; Li et al., 2011). Intrathecal injection of NR2B antagonists prior to spinal nerve ligation significantly inhibits the development of mechanical allodynia and can block the activity of wide dynamic range neurons in the spinal cord (Qu et al., 2009). We can speculate that changes in glutamatergic transmission following the neonatal low pH injections in the GN muscle could alter nociceptive processing through abnormal intracellular signaling cascades that later modulate the organization of pain circuits within the spinal cord. For example, the observed findings could relate to increased neuronal excitability from a decrease in inhibitory interneurons. Early overexpression of NMDAR resulting from increased afferent input, may lead to increased calcium fluxes and excitotoxicity of inhibitory interneurons in the spinal cord. This could cause disinhibition and ultimately hyperexcitability of second order neurons as observed in the current study. The increased expression of NR2B in the spinal cord also points to the potential involvement of NMDA receptors and long-term potentiation the spinal cord dorsal horn where the first sensory synapses are located. The increase in postsynaptic calcium influx would lead to potentiated excitatory synaptic transmission and spinal sensitization that at least in part explains behavioral hyperalgesia (Zhuo, 2009).
As previously defined, SL-A neurons demonstrate an increase in firing to CRD with an abrupt cessation of response after termination of the distending stimulus, while the SL-S neurons maintain a sustained response (> 4 s) following the distending stimulus (Ness and Gebhart, 1991; Ness and Gebhart, 1987). The SL-S neurons have been implicated to play more important role in sensitization based on the response characteristics. These include: 1) the firing response that outlasts the stimulus, 2) the absence of low-threshold convergent cutaneous inputs and 3) the presence of long ascending projections (Ness and Gebhart, 1987). We have demonstrated in our previous study that only SL-S, and not SL-A neurons, have altered responses to mechanical distension of the colon following the neonatal event (Miranda et al., 2006). Others have shown that acute colonic inflammation in adult rats increases the activity of SL-S and not SL-A spinal neurons (Ness and Gebhart, 2000). We have now shown that blocking the NMDARs decreases the responses of SL-S spinal neurons and not SL-A. Other investigators have shown that in naïve, adult rats without hyperalgesia, the AMPA receptor antagonist CNQX, differentially modified the activity of these two classes of neurons based on graded intensities of colon distension, having some effect on SL-A neurons (Ji and Traub, 2002). Another study in adult, naïve rats, demonstrated that the AMPA receptor antagonist DNQX, and not the NMDAR antagonist MK-801, decreased the responses of SL-A spinal neurons to CRD (Kozlowski et al., 2000). However, in a model of urinary bladder distension (Catroman and Ness 2002), three clinically available NMDAR antagonists (ketamine, dextromethorphan, and memantine) all demonstrated an inhibitory effect only on type I spinal neurons and not type II. Type I neurons, unlike type II, are subject to counter-irritation and are believed to be similar to SL-A neurons (Catroman and Ness 2002). While this would contradict the current findings, unlike studies using acute stimuli in adult rats, we focused on the role of these neuronal subtypes and the association with NMDARs in the development and maintenance of colonic hyperalgesia in a model of early life pain. Further, our classification of neurons only focused on sustained or abrupt spinal neurons and not counter-irritation to maintain consistency in our model (Peles et al., 2004; Miranda et al., 2006). Previous studies have suggested that these two neuronal subtypes may be influenced differently by the supraspinal inhibitory mechanism (Ness and Gebhart, 1991a; Ness and Gebhart, 1991b; Ness and Gebhart, 2001). Thus, a potential mechanism to explain the current results could be a decrease in the tonic inhibitory effect of the central opioidergic system (Tsuruoka and Willis et al., 1996). Modifications or permanent alterations can occur in supraspinal areas involved in descending inhibitory and/or facilitatory control that are immature during development. Thus, a window of vulnerability has been proposed where this system is move likely to develop aberrantly in the presence of pain (Hathway et al., 2012). While this could explain the hyperalgesia and increase in firing of spinal neurons in the current model, it does not entirely explain the differential effect on the neuronal subtypes.
Normal development of organized connections in the spinal cord resulting from competitive afferent input is disrupted by a repetitive painful stimulus that likely involves NMDARs. In the newborn rats, for example, the mechanoreceptive fields are large and gradually reduce in size over time (Torsney and Fitzgerald 2002). Blocking the NMDARs in rats during development results in a large cutaneous receptive field and lowers mechanical withdrawal thresholds (Beggs et al., 2002). Thus, the development and reorganization of the excitatory synaptic circuitry within the spinal cord may be a NMDAR dependent process that is significantly altered with a repetitive painful stimulus in areas of viscera-somatic convergence early in development. The role of spinal NMDARs in the development of hyperalgesia is further supported by a recent study demonstrating that chronic pain and the increase in expression of NR1 and NR2B following nerve injury can be prevented by NMDAR antagonist pre-treatment (Wilson et al., 2005). Further, the role of spinal NMDAR in maintaining the hyperalgesia is supported in the current study by demonstrating that intrathecal CGS-19755 decreases the response of awake, hyperalgesic rats to CRD but has no effect on naïve rats.
To investigate whether the site of action of the NMDA antagonist is limited to the CNS, we also tested the response characteristics of PNAs to mechanical distension before and after systemic NMDA antagonist administration. While others have found a role for primary sensory neurons in maintaining visceral hyperalgesia (Lin and Al-Chaer, 2003), we showed that at least in this model, peripheral NMDARs are unlikely to be involved in the maintenance of hyperalgesia since the response characteristics of PNAs were unchanged following CGS-19755. We deliberately avoided similar testing in naïve, control rats since no change was observed in the rats that demonstrated visceral hyperalgesia following pH 4.0 saline injections.
In summary, we have demonstrated that a subpopulation of spinal neurons (SL-S) are sensitized as a result of neonatal somatic pain and are influenced by NMDARs. The responses of primary sensory neurons to mechanical distension of the colon are not affected by NMDAR blockade and are unlikely to be involved in the later stages of visceral hyperalgesia. The results of this study, along with others, suggest that future pharmacological agents aimed at treated chronic visceral pain in children or adults should target the NR2B subunit of the NMDARs in the spinal cord.
Acknowledgments
Grant support: This work has been supported NIH K08 DK076198-01A1 grant awarded to Dr. Adrian Miranda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist with any of the authors.
References
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Zheng Y, Miller H, Miranda A, Sengupta JN, Shaker R. Alterations in N-methyl-D-aspartate receptor subunits in primary sensory neurons following acid-induced esophagitis in cats. Am J Physiol Gastrointest Liver Physiol. 2009;296:66–77. doi: 10.1152/ajpgi.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. PubMed. 2009 doi: 10.1111/pme.12939. PMID: 19254342. doi: 10.1038/tp.2013.43. [DOI] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Boxall SJ, Thompson SW, Dray A, Dickenson AH, Urban L. Metabotropic glutamate receptor activation contributes to nociceptive reflex activity in the rat spinal cord in vitro. Neuroscience. 1996;74:13–20. doi: 10.1016/0306-4522(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Brown KM, Wrathall JR, Yasuda RP, Wolfe BB. Quantitative measurement of glutamate receptor subunit protein expression in the postnatal rat spinal cord. Brain Res Dev Brain Res. 2002;137:127–133. doi: 10.1016/s0165-3806(02)00435-2. [DOI] [PubMed] [Google Scholar]
- Castroman PJ, Ness TJ. Ketamine, an N-methyl-D-aspartate receptor antagonist, inhibits the spinal neuronal responses to distension of the rat urinary bladder. Anesthesiology. 2002;96:1410–1419. doi: 10.1097/00000542-200206000-00021. [DOI] [PubMed] [Google Scholar]
- Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu X, Cao Z, Chen S, Owyang C, Li Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology. 2009;136:1732–1740. doi: 10.1053/j.gastro.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150:185–189. doi: 10.1016/j.bbr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Gaunitz C, Schuttler A, Gillen C, Allgaier C. Formalin-induced changes of NMDA receptor subunit expression in the spinal cord of the rat. Amino Acids. 2002;23:177–182. doi: 10.1007/s00726-001-0125-3. [DOI] [PubMed] [Google Scholar]
- Gonda X. Basic pharmacology of NMDA receptors. Curr Pharm Des. 2012;18:1558–1567. doi: 10.2174/138161212799958521. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Vega-Avelaira D, Fitzgerald M. A critical period in the supraspinal control of pain: opioid-dependent changes in brainstem rostroventral medulla function in preadolescence. Pain. 2012;153:775–783. doi: 10.1016/j.pain.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Takasusuki T, Yamaguchi S, Hori Y. NMDA receptor 2B subunit-mediated synaptic transmission in the superficial dorsal horn of peripheral nerve-injured neuropathic mice. Brain Res. 2007;1135:92–101. doi: 10.1016/j.brainres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Ji Y, Traub RJ. Differential effects of spinal CNQX on two populations of dorsal horn neurons responding to colorectal distension in the rat. Pain. 2002;99:217–222. doi: 10.1016/s0304-3959(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Kozlowski CM, Bountra C, Grundy D. The effect of fentanyl, DNQX and MK-801 on dorsal horn neurones responsive to colorectal distension in the anaesthetized rat. Neurogastroenterol Motil. 2000;12:239–247. doi: 10.1046/j.1365-2982.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006;29:219–228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Li S, Cao J, Yang X, Suo ZW, Shi L, Liu YN, Yang HB, Hu XD. NR2B phosphorylation at tyrosine 1472 in spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain hypersensitivity in mice. J Neurosci Res. 2011;89:1869–1876. doi: 10.1002/jnr.22719. [DOI] [PubMed] [Google Scholar]
- Li S, Cao J, Yang X, Suo ZW, Shi L, Liu YN, Yang HB, Hu XD. NR2B phosphorylation at tyrosine 1472 in spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain hypersensitivity in mice. J Neurosci Res. 2011;89:1869–1876. doi: 10.1002/jnr.22719. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Liu H, Cao Z, Chen S, Cao B, Liu J. Phosphorylated CaMKII post-synaptic binding to NR2B subunits in the anterior cingulate cortex mediates visceral pain in visceral hypersensitive rats. J Neurochem. 2012;121:662–671. doi: 10.1111/j.1471-4159.2012.07717.x. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain. 2010;149:555–564. doi: 10.1016/j.pain.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Peles S, Shaker R, Rudolph C, Sengupta JN. Neonatal nociceptive somatic stimulation differentially modifies the activity of spinal neurons in rats and results in altered somatic and visceral sensation. J Physiol. 2006;572:775–787. doi: 10.1113/jphysiol.2006.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil. 2011;23:683. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett. 2000;281:131–134. doi: 10.1016/s0304-3940(00)00832-6. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991;66:20–28. doi: 10.1152/jn.1991.66.1.20. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Inflammation enhances reflex and spinal neuron responses to noxious visceral stimulation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:649–657. doi: 10.1152/ajpgi.2001.280.4.G649. [DOI] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang BK, Collingridge GL. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130131. doi: 10.1098/rstb.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles S, Miranda A, Shaker R, Sengupta JN. Acute Nociceptive Somatic Stimulus Sensitizes Neurons in the Spinal Cord to Colonic Distension in the Rat. J Physiol. 2004;560:291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. NMDA Receptor Antagonists Attenuate Mechanical Hyperalgesia in Rats with Unilateral Inflammation of the Hindpaw. Neuroscience Letters. 1993;163:22–26. doi: 10.1016/0304-3940(93)90220-f. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald MJ. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. Neurophysiol. 2002;87:1311–1317. doi: 10.1152/jn.00462.2001. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Willis WD., Jr Descending modulation from the region of the locus coeruleus on nociceptive sensitivity in rat model of inflammatory hyperalgesia. Brain Res. 1996;743:86–92. doi: 10.1016/s0006-8993(96)01025-6. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Urch CE, Rahman W, Dickenson AH. Electrophysiological studies on the role of the NMDA receptor in nociception in the developing rat spinal cord. Developmental Brain Research. 2001;126:81–89. doi: 10.1016/s0165-3806(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Virgo L, Dekkers J, Mentis GZ, Navarrete R, de Belleroche J. Changes in expression of NMDA receptor subunits in the rat lumbar spinal cord following neonatal nerve injury. Neuropathol Appl Neurobiol. 2000;26:258–272. doi: 10.1046/j.1365-2990.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- Virgo L, Dekkers J, Mentis GZ, Navarrete R, de Belleroche J. Changes in expression of NMDA receptor subunits in the rat lumbar spinal cord following neonatal nerve injury. Neuropathol Appl Neurobiol. 2000;26:258–272. doi: 10.1046/j.1365-2990.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Garry EM, Anderson HA, Rosie R, Colvin LA, Mitchell R, Fleetwood-Walker SM. NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain. 2005;117:421–432. doi: 10.1016/j.pain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain. 2009;2:4–15. doi: 10.1186/1756-6606-2-4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]