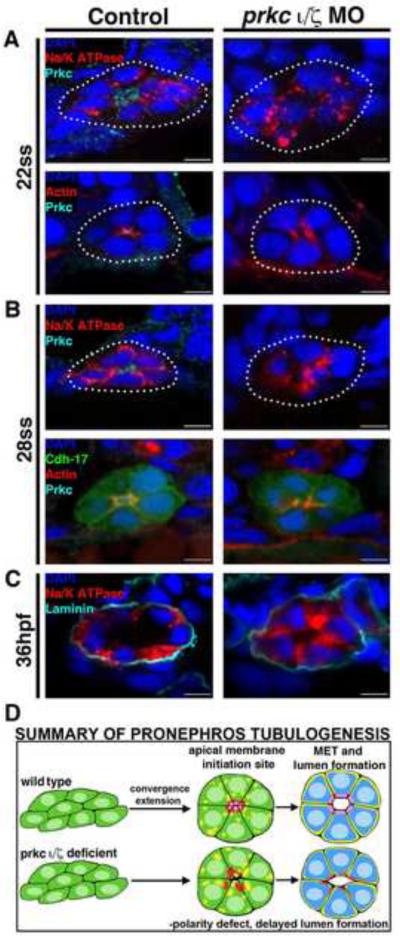
Figure 4. prkcι/ζ loss of function disrupts apical-basal protein localization and prevents proper polarity establishment, though delayed tubulogenesis eventually occurs.
Protein localization during pronephros development between the 22 ss through 36 hpf stages revealed alterations in double prkcι/ζ morphant nephrons compared to wild-type embryo controls. (A) At the 22 ss, double prkcι/ζ morphants had disorganized actin and lack Prkc at the apical domain, while Na+/K+ ATPase was diffusely spread over the entire cell membrane in both wild-types and double morphants. (B) At 28 ss, the Na+/K+ ATPase was still diffusely spread over the cell membrane in the morphants, but in wild-types this protein was restricted to the basolateral cell surface in a mutually exclusive domain to that of Prkcι/ζ and actin, which defined the apical domain. Double prkcι/ζ morphants continued to show disorganized actin and lack Prkcι/ζ at the 28 ss. (C) At 36 hpf, wild-type control and double prkcι/ζ morphant tubules were surrounded by the ECM component laminin. prkcι/ζ morphant embryos had unrestricted Na+/K+ ATPase and a single lumen was present, albeit less pronounced than in wild-type controls. (A-C) Scale bars, 5 μm. (D) Summary of pronephros tubulogenesis. Renal progenitors emerge as groups of mesenchymal cells that undergo rearrangements into circumferential clusters that display differential distribution of apical membrane components prior to lumen formation, at which time mutually exclusive domains of apical and basolateral proteins are established. Deficiency of Prkcι/ζ disrupts timely apical protein localization, delays lumen formation, and discrete apical and basolateral domains are not created.