Abstract
Maternal effects have gained attention as a method by which mothers may alter the physiological condition and phenotype of their offspring based upon current environmental conditions. The physiological and phenotypic outcomes of glucocorticoid-mediated maternal effects have been extensively studied in a variety of vertebrates; however, the underlying mechanism is currently unclear. Here, we injected tritiated corticosterone into the yolks of freshly laid Japanese quail eggs (Coturnix japonica) and traced its movement and metabolism through the in ovo development period. We found that corticosterone was extensively conjugated throughout the egg by the end of development, and while minimal corticosterone was detected within the embryo during development, accumulation of a conjugated metabolite in the embryo started to occur on day 6 of development. Because no movement and metabolism of corticosterone occurred in infertile eggs, our findings suggest that embryos are not passive recipients of maternal steroids, but instead appear to possess extensive metabolic capabilities, which may modulate their exposure to maternal steroids.
Keywords: corticosterone, maternal effect, metabolism, glucocorticoid, embryo, development
1. Introduction
The physiological state of a mother can impact the phenotype of her offspring through maternal effects [1]. Maternal effects are observed in a wide range of taxa, and represent a powerful method by which a mother may fine-tune offspring for current environmental conditions [1]. Steroids are widely conserved across vertebrates and represent one of the most studied mediators of maternal effects [2]. While studies on steroid-mediated maternal effects have demonstrated that steroids are capable of altering offspring phenotype, our understanding of the mechanisms through which these effects take place lags behind [3]. Specifically, the embryo's potential role in modulating steroid-mediated maternal effects has been often overlooked in favour of identifying phenotypic alterations (but see [4]). Characterizing how embryonic processes alter exposure to maternal steroids is vital to understanding the evolutionary consequences of maternal steroid effects.
Glucocorticoids (GCs) are a class of steroid hormones involved in metabolic homeostasis and the stress response in vertebrates [5]. Offspring that develop during periods of elevated maternal GCs have phenotypes that may increase short-term survival prospects, although long-term costs associated with GC hyper-exposure during development have been observed as well [6,7]. While this suggests that embryonic regulation of GCs is important to the life trajectory of an individual, until recently the embryo's role in regulating its exposure to GCs during development in in ovo systems has been minimally explored.
Developmental GC regulation in mammalian embryos has recently received attention as the long-term risk of developing many metabolic, physiological and behavioural disorders has been shown to increase after fetal hyper-exposure to GCs, especially synthetic GCs that mimic GC action, but are unable to be metabolized [8]. This suggests that the mammalian embryo relies upon its metabolic capabilities, often located in the placenta, to prevent such deleterious effects. Embryos of oviparous species are isolated from their mothers, making them an ideal subject for the study of developmental GC metabolism. Previous work on GCs in domestic chickens (Gallus gallus), and testosterone in European starlings (Sturnus vulgaris), has revealed that there is in ovo metabolism of yolk steroids [9,10]. However, this work focused on only early developmental stages, calling for a detailed characterization of how this metabolism impacts embryonic steroid exposure over the full course of development.
Here, we examine the movement and metabolism of corticosterone injected into the yolk of Japanese quail (Coturnix japonica) eggs by locating and characterizing corticosterone and its metabolites throughout the course of embryonic development. Rather than developing in a static environment, our results suggest the embryo plays a much more active role in the movement and metabolism of maternally derived steroids in the yolk than previously appreciated.
2. Material and methods
(a). Egg collection, treatment and incubation
Freshly laid eggs (n = 35) were collected from a breeding population of Japanese quail (C. japonica) maintained at Bucknell University. On the day of collection, each egg was injected, into the yolk, with a 5 μl bolus of sesame oil containing a small, physiological dose of approximately 0.4 ng of [1,2,6,7–3H]-corticosterone (PerkinElmer), the main avian GC. This amount was chosen based upon previous work that determined average endogenous corticosterone concentration to be 1.11 ± 0.30 ng g−1 [11]. Therefore, we raised corticosterone levels within normal physiological levels in order to avoid producing the phenotypic and physiological alterations due to GC-based maternal effects [6,7]. After injection, eggs were divided randomly into five groups (n = 5) with each egg in a group laid by a different female, and incubated for 3 (incubation day 3, ID3), 6 (ID6), 9 (ID9), 12 (ID12) or 15 (ID15) days to determine steroid movement and metabolism throughout the 18-day developmental period. Fertility rates were not 100% however, and final sample sizes for each group were ID3 = 5, ID6 = 3, ID9 = 4, ID12 = 4, ID15 = 3. In addition, unfertilized eggs were added to each incubation group (n = 2) to determine the effect of the developing embryo on the in ovo steroid environment. For incubation, freezer storage and other egg information, see the electronic supplementary material.
(b). Sample preparation for scintillation counter
Eggs were thawed at room temperature such that the extra-embryonic fluid, embryo and yolk could be separated, weighed and homogenized. While all egg components were collected from ID6, ID9 and ID12, ID3 samples contained no distinguishable embryo and ID15 samples contained no distinguishable extra-embryonic fluid. Yolk volume declined throughout development, and by ID15 yolk was not yet internalized into the embryo and was still recoverable. A 100 mg aliquot of each egg component was subjected to ether extraction to separate ether- and water-soluble corticosterone metabolites [10]. Aliquots of all samples were analysed in duplicate using a TriCarb-2910TR liquid scintillation counter.
(c). Thin layer chromatography
A more detailed characterization of corticosterone metabolites was conducted using thin layer chromatography (TLC). The remaining portions of homogenized yolk and embryo samples were extracted and concentrated before being separated into ether- and water-soluble portions. For a more detailed description of these procedures, see the electronic supplementary material. The ratio of ether- to water-soluble corticosterone for each developmental time point was determined by analysing a 20 μl aliquot of each sample. Samples were run in two solvent systems. The first separated free steroids (ether-soluble) from conjugated (water-soluble) steroids to determine whether conjugation had occured. The second separated the two major classes of steroid conjugates to reveal whether a conjugated steroid was a glucuronide or a sulfate. Plates were analysed following the methods of Paitz et al. [10] and standardized using the appropriate developmental time point ratio. For more details on the solvent systems and steroid standards used, see the electronic supplementary material.
3. Results
(a). Corticosterone movement
While the majority of injected corticosterone (approx. 90%) remained in the yolk by the end of development (ID15), a small portion of injected corticosterone (approx. 9%) was found in the embryo at ID15, illustrating corticosterone movement from the yolk to the embryo (figure 1). A transient drop in yolk radioactivity levels occured in all eggs on ID6 leading to an increase in extra-embryonic radioactivity levels (figure 1). This rise in extra-embryonic corticosterone was brief as levels declined steadily through the rest of development, coinciding with the loss of extra-embryonic fluid during the late stages of development. For infertile eggs, radioactivity remained in an ether-soluble form in the yolk throughout development.
Figure 1.
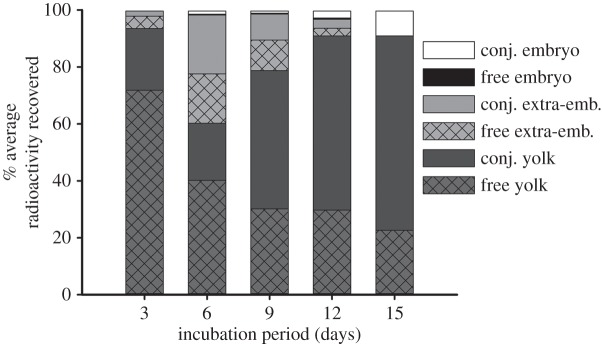
[3H]-Corticosterone was injected into fertile Japanese quail eggs and tracked for 3, 6, 9, 12 or 15 days of development. Free and conjugated levels of corticosterone in embryonic, extra-embryonic and yolk egg components were measured throughout development.
(b). Corticosterone metabolism
Over the course of development, injected corticosterone was metabolized to a conjugated form (figure 1). The relative amount of conjugated corticosterone compared with free corticosterone increased in both the yolk and embryo as development proceeded (figure 1). By ID15, 75% of yolk corticosterone and 100% of embryonic corticosterone was found in a conjugated form (figure 1).
(c). Thin layer chromatography
TLC performed on yolk samples confirmed that, while the majority of radioactive label migrated with the free corticosterone standard at ID6, by ID12 the migration pattern indicated the presence of a conjugated metabolite (figure 2a). Further TLC on the ID12 metabolite suggests the metabolite is a glucuronide [12,13] (figure 2b). Embryo samples from ID9, ID12 and ID15 showed similar patterns of migration compared with yolk samples, suggesting the corticosterone-conjugated metabolite in the embryo is identical to that in the yolk (electronic supplementary material, figure S1).
Figure 2.
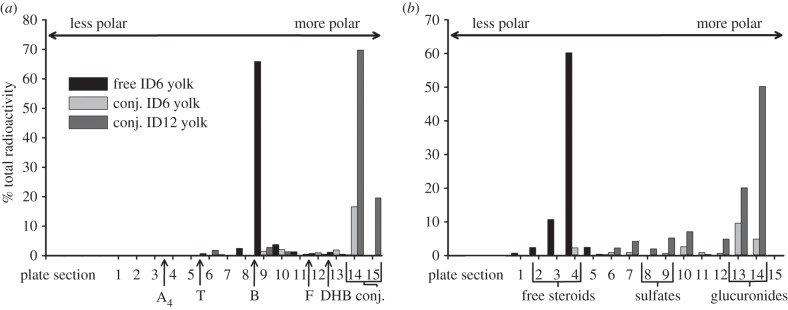
TLC allows for the separation of similar chemical species on a TLC plate based on differential migration rates. Plates were separated into 15 sections with plate section 1 representing the point of greatest migration (least polar) and plate section 15 the point of least migration (most polar). Samples were spotted onto section 15. Representative standardized TLC plates from ID6 and ID12 confirm the progressive metabolism of corticosterone in the yolk to a conjugated form. (a) The 95 : 5 solvent system separates free and conjugated steroids. Standards migrated according to polarity (A4, androstenedione; T, testosterone; B, corticosterone; F, cortisol; DHB, dihydrocorticosterone). (b) The 5 : 5 : 1 solvent system separates glucuronide conjugates from sulfate conjugates. Brackets indicate the migration pattern for free steroids, sulfate conjugates or glucuronide conjugates.
4. Discussion
Our results demonstrate for the first time that the embryo is exposed to maternal GCs that have been mostly conjugated and that most of the exposure to conjugated GCs occurs during the later stages of development. Specifically, by the end of incubation (ID15) approximately 80% of corticosterone in the egg was found in a conjugated form. Additionally, only conjugated corticosterone was observed in the embryo with levels increasing as development progressed (figure 1). Previous work examining both testosterone and corticosterone during the first third of development in chicken embryos revealed similar patterns of steroid movement and metabolism [9]. In addition, the transient drop we found in yolk radioactivity levels and increase in extra-embryonic radioactivity in early development was similar to that found in the chicken [9], suggesting this may be a normal pattern of in ovo steroid movement. However, by tracking corticosterone over the majority of the developmental period, we were able to examine the full extent of embryonic exposure to maternal GCs in ovo. Our findings demonstrate that the embryo metabolizes maternally derived corticosterone, potentially allowing for a measure of control over GC-mediated maternal effects.
Metabolism of maternal steroids has generally been regarded as a metabolic buffer [14], such that the embryo may altogether avoid the influence of maternal GCs or regulate its own exposure through selective reactivation. The almost complete metabolism of corticosterone suggests that our dose was low enough to be handled by this metabolic buffer. Embryonic hyper-exposure to GCs may occur when high levels of maternal GCs overwhelm the embryo's metabolic buffer, resulting in free GCs reaching the embryo and producing the phenotypes associated with GC-based maternal effects. Our work does not allow us to determine whether steroids conjugated in the yolk migrate to the embryo or if free steroids enter the embryo and then become conjugated. It is worth noting that conjugated metabolites themselves may alter development, as has been documented for oestradiol and oestrone sulfates in another oviparous vertebrate species [13,15]. To date, there has been no confirmed role for a conjugated form of GCs in either mammals or oviparous species and the conversion of conjugated metabolites back to free steroids may also be possible. The findings presented here suggest that answering these questions is necessary for a full understanding of GC-mediated maternal effects.
Our findings suggest that maternal effects result from a complex interaction of the original in ovo steroid environment and an embryo's metabolic capacity. Confirming previous studies [4], our work suggests that embryos may no longer be considered passive recipients of maternal steroids, but instead appear to possess extensive metabolic capabilities with which they modulate their exposure to maternal steroids. Owing to the lack of corticosterone conjugation in unfertilized eggs, our findings implicate embryonic initiation of conjugation, and it is currently unknown whether there is any maternal contribution. While the results presented here offer a more thorough picture of the developmental steroid environment the embryo is exposed to, further work is needed to understand if additional factors exist that can alter embryonic exposure to maternal steroids.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Bucknell Animal Care staff for their assistance and members of M.F.H.'s laboratory for helpful comments on a draft.
Ethics statement
All procedures were conducted with approval from the Bucknell University Institutional Animal Care and Use Committee.
Data accessibility
Data available from the Dryad Digital Repository: doi:10.5061/dryad.f57k3.
Funding statement
Funding was provided by an NSF grant (IOS-1145625) to M.F.H. and two awards to both B.G.V. and V.J.F. by the Beckman Scholars program and the Bucknell University Biology Department.
References
- 1.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 2.Gil D. 2008. Hormones in avian eggs: physiology, ecology and behavior. Adv. Stud. Behav. 38, 337–398. ( 10.1016/S0065-3454(08)00007-7) [DOI] [Google Scholar]
- 3.Moore MC, Johnston GIH. 2008. Toward a dynamic model of deposition and utilization of yolk steroids. Integr. Comp. Biol. 48, 411–418. ( 10.1093/icb/icn079) [DOI] [PubMed] [Google Scholar]
- 4.Reed WL, Clark ME. 2011. Beyond maternal effects in birds: responses of the embryo to the environment. Integr. Comp. Biol. 51, 73–80. ( 10.1093/icb/icr032) [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/edrv.21.1.0389) [DOI] [PubMed] [Google Scholar]
- 6.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriksen R, Rettenbacher S, Groothuis TGG. 2011. Prenatal stress in birds: pathways, effects, function, and perspectives. Neurosci. Behav. Rev. 35, 1484–1501. ( 10.1016/j.neubiorev.2011.04.010) [DOI] [PubMed] [Google Scholar]
- 8.Braun T, Challis JR, Newnham JP, Sloboda DM. 2013. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr. Rev. 34, 885–916. ( 10.1210/er.2013-1012) [DOI] [PubMed] [Google Scholar]
- 9.von Engelhardt N, Henriksen R, Groothuis TGG. 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocr. 163, 175–183. ( 10.1016/j.ygcen.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 10.Paitz RT, Bowden RM, Casto JM. 2011. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. B 278, 99–106. ( 10.1098/rspb.2010.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayward LS, Richardson JB, Grogan MN, Wingfield JC. 2006. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. Gen. Comp. Endocr. 146, 144–148. ( 10.1016/j.ygcen.2005.10.016) [DOI] [PubMed] [Google Scholar]
- 12.Sarfaty GA, Lipsett MB. 1966. Separation of free and conjugated 11-deoxy-17-oxosteroids by thin-layer chromatography. Analyt. Biochem. 15, 184–186. ( 10.1016/0003-2697(66)90267-3) [DOI] [PubMed] [Google Scholar]
- 13.Paitz RT, Bowden RM. 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol. 53, 895–901. ( 10.1093/icb/ict027) [DOI] [PubMed] [Google Scholar]
- 14.Seckl JR, Holmes MC. 2007. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 3, 479–488. ( 10.1038/ncpendmet0515) [DOI] [PubMed] [Google Scholar]
- 15.Paitz RT, Bowden RM. 2010. Biological activity of oestradiol sulphate in an oviparous amniote: implications for maternal steroid effects. Proc. R. Soc. B 278, 2005–2010. ( 10.1098/rspb.2010.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: doi:10.5061/dryad.f57k3.


