Abstract
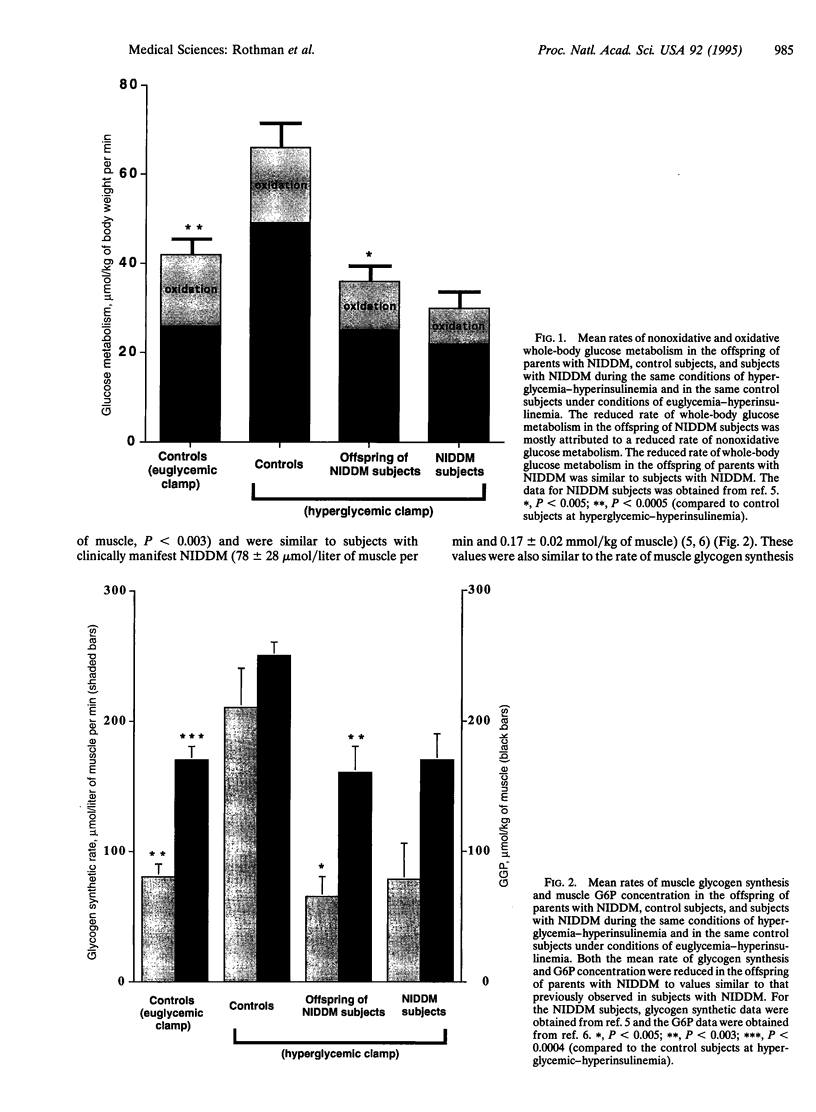
Recent studies have demonstrated that reduced insulin-stimulated muscle glycogen synthesis is the major cause of insulin resistance in patients with non-insulin-dependent diabetes mellitus (NIDDM). This reduced rate has been assigned to a defect in either glucose transport or hexokinase activity. However it is unknown whether this is a primary or acquired defect in the pathogenesis of NIDDM. To examine this question, we measured the rate of muscle glycogen synthesis and the muscle glucose 6-phosphate (G6P) concentration using 13C and 31P NMR spectroscopy as well as oxidative and nonoxidative glucose metabolism in six lean, normoglycemic offspring of parents with NIDDM and seven age/weight-matched control subjects under hyperglycemic (approximately 11 mM)-hyperinsulinemic (approximately 480 pM) clamp conditions. The offspring of parents with NIDDM had a 50% reduction in total glucose metabolism, primarily due to a decrease in the nonoxidative component. The rate of muscle glycogen synthesis was reduced by 70% (P < 0.005) and muscle G6P concentration was reduced by 40% (P < 0.003), which suggests impaired muscle glucose transport/hexokinase activity. These changes were similar to those previously observed in subjects with fully developed NIDDM. When the control subjects were studied at similar insulin levels (approximately 440 pM) but euglycemic plasma glucose concentration (approximately 5 mM), both the rate of glycogen synthesis and the G6P concentration were reduced to values similar to the offspring of parents with NIDDM. We conclude that insulin-resistant offspring of parents with NIDDM have reduced nonoxidative glucose metabolism and muscle glycogen synthesis secondary to a defect in muscle glucose transport/hexokinase activity prior to the onset of overt hyperglycemia. The presence of this defect in these subjects suggests that it may be the primary factor in the pathogenesis of NIDDM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson K., Galuska D., Thörne A., Sonnenfeld T., Wallberg-Henriksson H. Decreased insulin-stimulated 3-0-methylglucose transport in in vitro incubated muscle strips from type II diabetic subjects. Acta Physiol Scand. 1991 Jun;142(2):255–260. doi: 10.1111/j.1748-1716.1991.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Bloch G., Chase J. R., Avison M. J., Shulman R. G. In vivo 31P NMR measurement of glucose-6-phosphate in the rat muscle after exercise. Magn Reson Med. 1993 Sep;30(3):347–350. doi: 10.1002/mrm.1910300311. [DOI] [PubMed] [Google Scholar]
- Bloch G., Chase J. R., Meyer D. B., Avison M. J., Shulman G. I., Shulman R. G. In vivo regulation of rat muscle glycogen resynthesis after intense exercise. Am J Physiol. 1994 Jan;266(1 Pt 1):E85–E91. doi: 10.1152/ajpendo.1994.266.1.E85. [DOI] [PubMed] [Google Scholar]
- Bonadonna R. C., Del Prato S., Saccomani M. P., Bonora E., Gulli G., Ferrannini E., Bier D., Cobelli C., DeFronzo R. A. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest. 1993 Jul;92(1):486–494. doi: 10.1172/JCI116592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990 Oct;70(4):1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Thuillez P., Lillioja S., Zawadzki J., Bogardus C. Insulin sensitivity in adipocytes from subjects with varying degrees of glucose tolerance. Am J Physiol. 1986 Sep;251(3 Pt 1):E306–E310. doi: 10.1152/ajpendo.1986.251.3.E306. [DOI] [PubMed] [Google Scholar]
- Freymond D., Bogardus C., Okubo M., Stone K., Mott D. Impaired insulin-stimulated muscle glycogen synthase activation in vivo in man is related to low fasting glycogen synthase phosphatase activity. J Clin Invest. 1988 Nov;82(5):1503–1509. doi: 10.1172/JCI113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991 Mar;87(3):1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R., Prolla T. A., Shulman R. G. 13C NMR visibility of rabbit muscle glycogen in vivo. Magn Reson Med. 1991 Aug;20(2):327–332. doi: 10.1002/mrm.1910200216. [DOI] [PubMed] [Google Scholar]
- Gulli G., Ferrannini E., Stern M., Haffner S., DeFronzo R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes. 1992 Dec;41(12):1575–1586. doi: 10.2337/diab.41.12.1575. [DOI] [PubMed] [Google Scholar]
- Halvorson H. R., Vande Linde A. M., Helpern J. A., Welch K. M. Assessment of magnesium concentrations by 31P NMR in vivo. NMR Biomed. 1992 Mar-Apr;5(2):53–58. doi: 10.1002/nbm.1940050202. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Katz A., Raz I., Spencer M. K., Rising R., Mott D. M. Hyperglycemia induces accumulation of glucose in human skeletal muscle. Am J Physiol. 1991 Apr;260(4 Pt 2):R698–R703. doi: 10.1152/ajpregu.1991.260.4.R698. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Howard B. V., Bennett P. H., Yki-Järvinen H., Freymond D., Nyomba B. L., Zurlo F., Swinburn B., Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988 May 12;318(19):1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Young A. A., Culter C. L., Ivy J. L., Abbott W. G., Zawadzki J. K., Yki-Järvinen H., Christin L., Secomb T. W., Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987 Aug;80(2):415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Piras R., Staneloni R. In vivo regulation of rat muscle glycogen synthetase activity. Biochemistry. 1969 May;8(5):2153–2160. doi: 10.1021/bi00833a056. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson S., Edelson D., Cerasi E. In vitro autoregulation of glucose utilization in rat soleus muscle. Diabetes. 1987 Sep;36(9):1041–1046. doi: 10.2337/diab.36.9.1041. [DOI] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Härkonen M., Groop L. C. Impaired activation of glycogen synthase in people at increased risk for developing NIDDM. Diabetes. 1992 May;41(5):598–604. doi: 10.2337/diab.41.5.598. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Taylor R., Price T. B., Rothman D. L., Shulman R. G., Shulman G. I. Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med. 1992 Sep;27(1):13–20. doi: 10.1002/mrm.1910270103. [DOI] [PubMed] [Google Scholar]
- Warram J. H., Martin B. C., Krolewski A. S., Soeldner J. S., Kahn C. R. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990 Dec 15;113(12):909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Helve E., Koivisto V. A. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes. 1987 Aug;36(8):892–896. doi: 10.2337/diab.36.8.892. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Young A. A., Lamkin C., Foley J. E. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987 Jun;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Stone K., Mott D. M. Insulin response of components of whole-body and muscle carbohydrate metabolism in humans. Am J Physiol. 1988 Feb;254(2 Pt 1):E231–E236. doi: 10.1152/ajpendo.1988.254.2.E231. [DOI] [PubMed] [Google Scholar]