Figure 2.
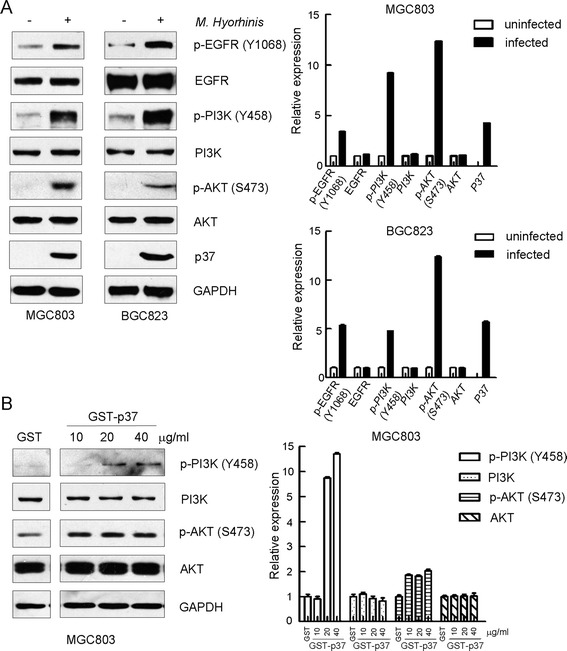
Both M. hyorhinis and p37 activate PI3K-AKT signaling. (A) M. hyorhinis upregulates EGFR, PI3K and AKT phosphorylations in gastric cancer cell MGC803 and BGC823. Cells were serum starved for 24 hours and then infected with M. hyorhinis for another 2 hours. Protein lysates were analyzed by Western blot with indicated antibodies. (B) Purified p37 protein upregulates PI3K and AKT phosphorylations in gastric cancer cell MGC803. Cells were serum starved for 24 hours and then treated with p37 for another 2 hours. Protein lysates were analyzed by Western blot with indicated antibodies. Optical densities of protein bands were quantified by Image J software and relative expression levels of indicated protein to loading control were shown in graph. Values represented the mean ± SD from three to four independent experiments.
