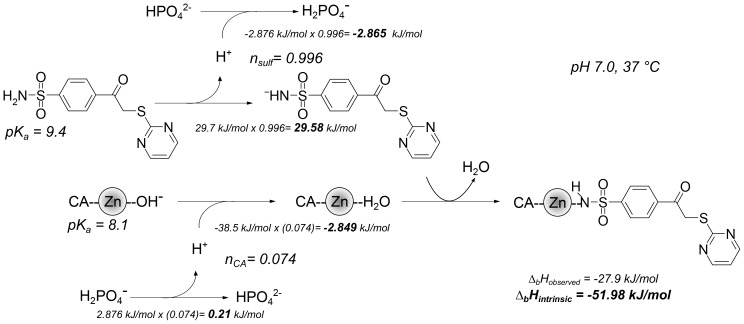
Figure 7. The processes linked to the binding of 3b to CA I.
The two left-central reactions show the binding-linked deprotonation of the inhibitor sulfonamide and the protonation of the zinc-bound hydroxide, respectively. Top and bottom lines show linked phosphate buffer (de)protonation reactions. The numbers give estimates of the enthalpies for each process multiplied by the number of linked protons (n) yielding the observed enthalpic contribution of each reaction at pH 7.0, 37°C. The intrinsic enthalpy of binding, shown by the rightmost arrow, is equal to −51.98 kJ/mol. The observed enthalpy, estimated for phosphate buffer at pH 7.0, is equal to −27.90 kJ/mol. Zinc atom is shown as grey shaded sphere and the carbonic anhydrase protein is shown as CA.